Elastin integrity in bicuspid valve-associated aortopathy is associated with altered biomechanical properties and influenced by age
Introduction
Bicuspid aortic valve (BAV) disease is the most common congenital heart defect and affects one in every 50 persons (1). Progressive weakening of the ascending aorta in these patients (i.e., aortopathy), places them at an increased risk of aortic dissection or rupture (2). The mechanisms underlying this aortopathy are unclear. Abnormal histopathology within the BAV aortic wall is a well-accepted macroscopic manifestation of microscopic extracellular matrix changes mediated by cellular proteins including matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) (1,3). Our group and others previously showed that valve-mediated hemodynamics influence the expression of aortic remodeling in BAV-associated aortopathy (4-10), and that regional differences in hemodynamics predict both the regional expression of histopathological disease within these aortas as well as degree of elastin fiber derangement—a key mediator of aortic wall mechanical properties (8,9,11).
In a small subset of patients, we found that greater elastin dysfunction corresponded to increased stiffness, a mechanical metric that has been previously linked to aortic disease (9). However, this was a limited pilot study. The influence of histopathological derangement of elastin fibers and their consequences on aortic wall biomechanics in BAV-associated aortopathy remain unclear. Furthermore, the influence of age in histopathological and biomechanical studies of BAV patients are poorly defined.
Given that aortic diameter alone is an inadequate criterion to guide surgical management of these patients (12-14), understanding the mechanical properties of the BAV aorta will help further clarify how to best address this cardiovascular pathology.
The present study sought to compare the relationship of elastin fiber integrity with ex vivo mechanical properties representing both physiological and supraphysiological mechanical loading of ascending aortic tissue from patients with BAV aortopathy in the 6th and 7th decades of life (51–70 years).
Methods
Patients
Nineteen patients undergoing elective prophylactic ascending aortic resection at the Foothills Medical Centre (Calgary, Alberta, Canada) and Northwestern Memorial Hospital (Chicago, IL, USA) between April 2016 and January 2020 were enrolled in this study. All BAV patients were greater than 18 years of age and had no prior history of aortic surgery, dissection, rupture, or connective tissue disorder. This study was conducted with the approval of the of the Conjoint Faculties Research Ethics Board at the University of Calgary (REB14-0084) and the Northwestern University Institutional review board (STU00204434) with written informed consent obtained from all participants. Demographic data such as age, sex, aneurysm size, and history of hypertension was also collected. The degree of stenosis and regurgitation were determined through continuous-wave Doppler ultrasound and graded based on clinical guidelines (15).
Data collection
Tissue samples were collected as permitted by the extent of the ascending aorta resection. The resected tissue was subdivided into circumferential regions: anterior, greater curvature, posterior, and lesser curvature. These regions were further divided into ex vivo mechanical testing specimens (12 mm × 12 mm square) with the circumferential and axial orientations noted. The histology samples were collected from the ex vivo specimens after mechanical testing. Specimens were flash frozen in VWR tissue freezing compound and stored at −80 ℃ until testing. When the circumferential region was too small for mechanical specimen to be obtained, the specimen was excluded from the study.
The mechanical testing of the tissue was performed as previously described (16). Specimens were subject to planar biaxial testing (ElectroForce System, TA Instruments, Springfield MO, with two 22N load cells). Specimens were pre-loaded to 0.05N then subjected to a series of displacement-controlled testing protocols (1:1, 1:0.5, 0.5:1; where ‘1’ indicates a maximum of 60% displacement based on the specimen’s edge length). Tissue deformation was tracked through five dots placed in the central region of the sample; force was recorded by the testing device in the two directions. Mechanical behaviour was determined from the deformation gradient under the assumption of tissue homogeneity and incompressibility. Measures were taken in the testing set up and post-processing to ensure shear stress was low enough to be considered negligible. Cauchy stress and stretch were determined via the deformation gradient from the local dot tracking, the measured forces, and the dimensions of the specimens for each protocol. For better comparison across specimens, each of the resulting stress-strain relationships from the same specimen were fitted to surface representing the mechanical behaviour of that specimen (16). From this surface, the true equi-stretch (1:1 stretch ratio) mechanical behaviour was obtained. Four mechanical parameters were determined from the equi-stretch behaviour. A tangential modulus, similar to a stiffness measure, was determined for each linear region of the behaviour. A low-strain tangential modulus (LTM) was determined from the initial linear region. If a second linear region was present after the transition zone, a high-strain tangential modulus (HTM) was calculated. For specimens displaying a non-linear transition region, the onset and end stresses (TZo and TZe, respectively) were recorded. For the present study, LTM and TZo were defined as metrics of physiological loading conditions, while TZe and HTM were those of supraphysiological loading conditions.
For histological analysis, tissue was formalin-fixed and paraffin-embedded prior to 5 µm sectioning. Musto-Movat pentachrome staining was performed, and entire tissue sections digitized with Aperio ImageScope digital scanning (Leica Biosystems, Aperio ImageScope, Version 12.4.3.5008) for colorimetric analysis of the aortic media elastin. Each specimen was also graded by a pathologist for level of elastin fragmentation in accordance with the consensus statement for evaluation of non-inflammatory aortopathy from the Society for Cardiovascular Pathology (17).
Statistical analysis
A custom statistical analysis Python software pipeline (Python Software Foundation, https://www.python.org/) was developed using the SciPy, Pingouin, and Statsmodel packages (18-21). Descriptive statistics were presented as mean ± standard deviation. The significance threshold was set to 0.05 for all tests. A Shapiro-Wilk test was implemented to assess the normality of each continuous variable. For non-normally distributed data, a Box-Cox transformation was applied to ensure normality. To assess the equivalence of variance among the mechanical properties and elastin fragmentation a Levene test was performed. To determine statistically significant relationships among the mechanical properties and the elastin quantities, a linear mixed effect model was used with inter-patient variability set as a random effect. The normality and heteroscedasticity of the residuals of the models were assessed through the Shapiro-Wilk and the White test respectively. P values are presented along with the Marginal Coefficient of Determination which is the ratio of variance explained by the explanatory variable as comparted to the total variance in the data. The graphs presented in this study were created using the Plotnine package. Relationships between the mechanical properties and elastin were examined with respect to the total population and in age categories based on decades (i.e., 51–60 and 61–70 years old). Mechanical and elastin properties were also correlated with diameter size via a Spearman correlation due to the non-linearity of the diameter data even after a Box-Cox transformation
Results
Patient characteristics
A total of 66 aortic tissue samples were collected from 19 patients: 10 patients were categorized as the younger population group (i.e., 51–60 years at the time of aortic resection) and nine were categorized as the older population group (i.e., 61–70 years). Patient demographics and preoperative characteristics are summarized in Table 1. There were significant differences in mid ascending aneurysm size between the younger and older population (P=0.034) with the younger age group having a larger diameter, however there was no significant difference between the age groups for the Sinus of Valsalva. There were also no significant differences between mechanical properties or elastin properties and diameter size within each age group.
Table 1
| Clinical characteristics | Age group 51–60 years (N=10 patients) | Age group 61–70 years (N=9 patients) |
|---|---|---|
| Age (years) ± SD | 56±2 | 64±3 |
| Female, n [%] | 1 [10] | 0 [0] |
| BAV classification, n [%] | ||
| Type 1, RN | 2 [20] | 3 [33] |
| Type 1, RL | 5 [50] | 5 [55] |
| Type 2, RL/RN | 2 [20] | 0 [0] |
| Other | 1 [10] | 1 [11] |
| Aortic valve function, n [%] | ||
| AS: none/mild/moderate-severe | 2 [20]/1 [10]/7 [70] | 1 [11]/3 [33]/5 [55] |
| AR: none/mild/moderate-severe | 2 [20]/3 [30]/5 [50] | 4 [44]/3 [33]/2 [22] |
| Ascending aorta diameter (cm), mean ± SD | ||
| Sinus of Valsalva | 4.1±0.5 | 4.6±0.4 |
| Mid ascending aorta | 5.1±0.2 | 4.7±0.4 |
| Cardiovascular risk factors, n [%] | ||
| Hypertension | 2 [20] | 2 [22] |
| Diabetes | 0 [0] | 1 [11] |
| Surgical procedure: aortic valve, n [%] | ||
| Repair | 0 [0] | 0 [0] |
| Replacement | 9 [90] | 9 [100] |
| None | 1 [10] | 0 [0] |
| Surgical procedure: ascending aorta, n [%] | ||
| Ascending aorta replacement | 7 [70] | 4 [44] |
| Root replacement | 0 [0] | 0 [0] |
| Hemi-arch | 3 [30] | 5 [56] |
Data presented as mean ± SD or number [%]. Comparison between age groups (Student t-test) indicated with P values. SD, standard deviation; BAV, bicuspid aortic valve; RN, right-noncoronary cusp fusion; RL, right-left coronary cusp fusion; AS, aortic stenosis; AR, aortic regurgitation.
Age group differences
A comparison between each elastin and mechanical property between the two age groups can be found in Table 2. While all the mechanical properties were greater for the older age group, no statistically significant differences between the older and younger BAV patients were appreciated. Similarly, no statistically significant difference in elastin content was noted between age groups, although semi-quantitative scoring of elastin fragmentation demonstrated that a greater proportion of the older BAV patients exhibited mild or moderate disease compared to their younger counterparts (Table 2).
Table 2
| Aortic wall tissue characteristic | Age group 51–60 years (N=36 samples) | Age group 61–70 years (N=30 samples) | P value |
|---|---|---|---|
| Histopathology | |||
| Elastin content (%), mean ± SD | 35±6 | 33±6 | 0.780 |
| Elastin fragmentation score, n [%] | |||
| Absent | 14 [39] | 7 [22] | – |
| Mild | 19 [53] | 19 [61] | – |
| Moderate | 3 [8] | 4 [13] | – |
| Circumferential biomechanics (kPa), mean ± SD | |||
| LTM | 429±105 | 447±137 | 0.670 |
| TZo | 31±9 | 40±20 | 0.193 |
| TZe | 93±31 | 106±37 | 0.420 |
| HTM | 1,261±734 | 1,604±898 | 0.390 |
| Axial biomechanics (kPa), mean ± SD | |||
| LTM | 342±112 | 356±108 | 0.520 |
| TZo | 24±8 | 34±16 | 0.075 |
| TZe | 67±28 | 82±29 | 0.120 |
| HTM | 956±781 | 1,378±790 | 0.058 |
Data presented as mean ± SD or number [%] for aortic wall tissue samples. Comparison between age groups (Student t-test) indicated with P values. SD, standard deviation; LTM, low strain modulus; TZo, transition zone onset stress; TZe, transition zone end stress; HTM, high strain modulus.
Elastin and physiological mechanics
No statistically significant relationships between the elastin content in all BAV patients and LTM and TZo were noted, but significant differences emerged upon the stratification by young and older age groups (Figure 1). In the younger BAV population, LTM and elastin content were positively correlated such that greater elastin content corresponded with higher LTM (Figure 1A; P=0.032, marginal R2=0.694). In contrast, this correlation was negative for the older BAV population where greater elastin content corresponded with a lower LTM (Figure 1B; P=0.037, marginal R2=0.95). Both populations also demonstrated significant associations between circumferential LTM and elastin fragmentation; younger BAV patients exhibited lower LTM where fragmentation was present compared to those without fragmentation (Figure 1C; P=0.005, absent to mild), whereas older BAV patients demonstrated lower LTM in samples without fragmentation (Figure 1D; P=0.049, absent to mild). For the axial direction, only the older population showed a significant relationship between LTM and elastin content; LTM was significantly higher when elastin fragmentation was present (P=0.047, absent to mild). Circumferential TZo was associated with elastin fragmentation in both BAV age groups. Younger BAV patients demonstrated lower TZo alongside increased elastin fragmentation (Figure 1E; P=0.044, absent to mild), while older BAV patients exhibited increased TZo with increased fragmentation (Figure 1F; P=0.001, absent to mild; P=0.049, absent to moderate).
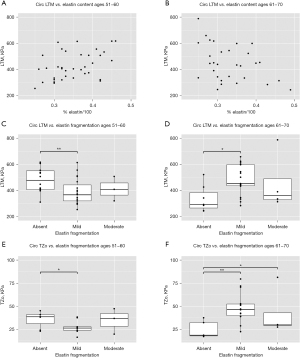
Elastin and supraphysiological mechanics
Statistically significant relationships between measures of elastin integrity and supraphysiological mechanical properties were only appreciated in the older BAV patients (Figure 2). Elastin content inversely correlated with circumferential TZe (Figure 2B, P=0.049, marginal R2=0.98), and worsened elastin fragmentation corresponded with increased circumferential HTM (Figure 2D, P<0.001 absent to mild, P=0.001 absent to moderate). No statistically significant associations were observed between histopathology and axial supraphysiological mechanical properties.
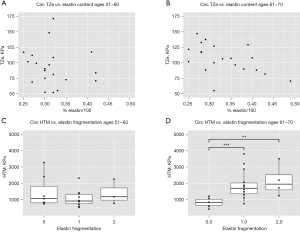
Discussion
BAV aortopathy is characterized by elastin fiber degeneration within the aortic wall, as well as aberrant proximal aortic biomechanics (3,22,23). However, the relationship between the two, especially with respect to age remain incompletely defined. Most tissue studies group all BAV patients together despite studies documenting the need for more nuanced stratification of this heterogeneous patient population. Using 66 tissue samples from 19 BAV patients, we compared the relationship between elastin fiber integrity and ex vivo biomechanical properties from patients undergoing ascending aortic surgery. We report that: (I) abnormal elastin histopathology corresponds with altered biomechanics in the proximal BAV aorta, and; (II) this relationship differs by age (i.e., 51–60 years old versus 61–70 years old) and with respect to physiological and supraphysiological mechanical properties.
Heterogeneous aortic wall remodeling is well-documented in the BAV aorta (5-10). Compared to tricuspid aortic valve (TAV), histopathological studies support a unique remodeling process in the BAV aortic wall where heterogeneous expression of disease corresponds with both the cusp fusion pattern as well as valve-mediated hemodynamics (4,6-9,11). In the present study, we similarly found variable elastin fragmentation in the BAV aortic wall and build on previous findings by documenting differences in elastin integrity between BAV patients in their sixth and seventh decades of life (i.e., “younger” and “older” patients, respectively). These changes in elastin are in turn associated with differences in ex vivo aortic wall mechanical properties. These properties are primarily dictated by elastin and collagen fibers with the former governing mechanical behaviour in a low strain region (characterized by LTM) prior to collagen recruitment (TZo), and facilitates large deformations under physiological loading conditions. In contrast, collagen bears higher loads at supraphysiological conditions and is only engaged when a critical threshold of strain is reached (24). This results in a non-linear transition region leading to fully-engaged collagen fibers (TZe) and a second linear region that is predominantly collagen-mediated (HTM).
In the present study with respect to physiological material properties, we report that decreased elastin fiber integrity in the younger group of BAV patients was associated with a decrease in both LTM and TZo, suggestive of decreased stiffness and a lower threshold for collagen fiber recruitment for mechanical loading, respectively. By contrast, decreased elastin fiber integrity was associated with an increased LTM and TZo in the older BAV patients, consistent with increased aortic wall stiffness. Furthermore, compromised elastin integrity in older BAV patients also corresponded with supraphysiological material properties consistent with a higher threshold for complete collagen fiber engagement (i.e., elevated TZe) alongside increased stiffening (i.e., increased HTM). These divergent findings between younger and older BAV patients could potentially be attributed to the aging effects with the biomechanics of the older patients being affected by both disease progression and aging. Given the natural history of arterial aging, elastin gradually breaks down without compensatory replacement, and collagen fibers become the predominant mechanical bearing unit of the aortic wall (23,25). The result is an overall stiffening of arteries that is itself an independent predictor of aortic dilatation and the need for aortic surgery (26-28), as well as a key driver of aortopathy pathogenesis (29-32). Our data suggest that the mechanical properties of the older patients follow this aging effect with a stiffening associated with elastin breakdown occurring in both the low-strain (LTM) and high-strain regions (HTM). Interestingly, in the younger group, elastin fiber breaks down more readily and leads to aortic wall compliance. Increased compliance has been previously associated with aortic histopathologic alterations and disease progression but has yet to be associated with any aging effects (33). Aortopathy has been considered a form of accelerated vascular aging as characterized by elastin degradation, reduction in smooth muscle cell number and contractile capacity as well as increased collagen content (34,35). However, our study suggests that the effect on the resulting biomechanics may be more complex with the interplay between aortic disease and aging. Given that the BAV aortic wall exhibits considerable regional heterogeneity in enzymatic activity and histopathology (5-10), both of which are influenced by valve-mediated hemodynamics that is itself influenced by patient age (36), our data provides a direct interrogation of the elastin integrity and mechanical properties, and build on previous work by showing that these relationships in the BAV aorta are further influenced by age, and warrant more nuanced stratification of this heterogeneous patient population.
Several limitations for this work exist. First, although a substantial number of tissue samples were analyzed, they were derived from only 19 separate patients. Future studies already underway will include a greater number of patients from a variety of age groups. Second, no comparison of the present data to TAV aortic wall in either health or disease was carried out. Future interrogations using these controls will be important to clarify whether the relationship between elastin and mechanical properties observed in this study are similar in non-BAV aortic wall. Third, given the important role of collagen in governing aortic wall biomechanics and the dynamic behaviour of the aorta, these data were limited to only elastin. Combined elastin and collagen histopathology analysis with mechanical testing is warranted. Lastly, the mechanical testing employed in this paper is limited by its ex vivo approach, and the interactions between the natural stresses of tissue in vivo as well as the living cells within the aortic wall are not accounted for in our analysis. Further validation with in vivo aortic mechanics is needed.
In summary, elastin disruption in the BAV aorta is associated with ex vivo mechanical behaviour of aortic tissue, and this relationship differs based on BAV patient age and with respect to physiological and supraphysiological mechanical properties. These novel data further our understanding of the influence of elastin fiber integrity on aortic biomechanics and support age-related considerations for future studies of BAV patients. Moreover, this work encourages nuanced assessment of BAV patients as a heterogeneous patient population. Further study is warranted to delineate how these mechanical properties can best direct the surgical management of BAV aortopathy.
Acknowledgments
Funding: This work was supported by National Institutes of Health grant (NIH HL133504 AJB, ESDM, PWMF), NSERC Discovery Programme (ESDM), an Alberta Innovates Health Solutions MD-PhD Studentship (DGG), and by the Melman Bicuspid Aortic Valve Program, Bluhm Cardiovascular Institute (PWMF).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920-9. [Crossref] [PubMed]
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104-12. [Crossref] [PubMed]
- Fedak PW, de Sa MP, Verma S, et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg 2003;126:797-806. [Crossref] [PubMed]
- Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014;129:673-82. [Crossref] [PubMed]
- Kotlarczyk MP, Billaud M, Green BR, et al. Regional Disruptions in Endothelial Nitric Oxide Pathway Associated With Bicuspid Aortic Valve. Ann Thorac Surg 2016;102:1274-81. [Crossref] [PubMed]
- Della Corte A, Quarto C, Bancone C, et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell-matrix signaling. J Thorac Cardiovasc Surg 2008;135:8-18, 18.e1-2.
- Tsamis A, Phillippi JA, Koch RG, et al. Extracellular matrix fiber microarchitecture is region-specific in bicuspid aortic valve-associated ascending aortopathy. J Thorac Cardiovasc Surg 2016;151:1718-28.e5. [Crossref] [PubMed]
- Guzzardi DG, Barker AJ, van Ooij P, et al. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights From Wall Shear Stress Mapping. J Am Coll Cardiol 2015;66:892-900. [Crossref] [PubMed]
- Bollache E, Guzzardi DG, Sattari S, et al. Aortic-valve mediated wall shear stress is heterogeneous and predicts regional aortic elastic fiber thinning in bicuspid aortic valve-associated aortopathy. J Thorac Cardiovasc Surg 2018;156:2112-20.e2. [Crossref] [PubMed]
- McClarty D, Ouzounian M, Tang M, et al. Ascending aortic aneurysm haemodynamics are associated with aortic wall biomechanical properties. Eur J Cardiothorac Surg 2022;61:367-75. [Crossref] [PubMed]
- Soulat G, Scott MB, Allen BD, et al. Association of Regional Wall Shear Stress and Progressive Ascending Aorta Dilation in Bicuspid Aortic Valve. JACC Cardiovasc Imaging 2022;15:33-42. [Crossref] [PubMed]
- Pape LA, Tsai TT, Isselbacher EM, et al. Aortic Diameter ≥5.5 cm Is Not a Good Predictor of Type A Aortic Dissection: Observations From the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116:1120-7. [Crossref] [PubMed]
- Hardikar AA, Marwick TH. The natural history of guidelines: the case of aortopathy related to bicuspid aortic valves. Int J Cardiol 2015;199:150-3. [Crossref] [PubMed]
- Gregory AJ, Di Martino E, Fedak PWM. Aortic diameter: The beginning of the end of an era. J Thorac Cardiovasc Surg 2018;156:513-4. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Forneris A, Nightingale M, Ismaguilova A, et al. Heterogeneity of Ex vivo and In vivo Properties along the Length of the Abdominal Aortic Aneurysm. Appl Sci 2021;11:3485. [Crossref]
- Halushka MK, Angelini A, Bartoloni G, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases - nomenclature and diagnostic criteria. Cardiovasc Pathol 2016;25:247-57. [Crossref] [PubMed]
- Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 2020;17:261-72. [Crossref] [PubMed]
- Vallat R. Pingouin: statistics in Python. J Open Source Softw 2018;3:1026. [Crossref]
- Seabold S, Perktold J. Statsmodels: Econometric and Statistical Modeling with Python. Python in Science Conference, 2010:92-6.
- Van Rossum G, Drake F. Python 3 Reference Manual. Scotts Valley, CA: CreateSpace, 2009.
- Wu D, Shen YH, Russell L, et al. Molecular mechanisms of thoracic aortic dissection. J Surg Res 2013;184:907-24. [Crossref] [PubMed]
- Emmott A, Garcia J, Chung J, et al. Biomechanics of the Ascending Thoracic Aorta: A Clinical Perspective on Engineering Data. Can J Cardiol 2016;32:35-47. [Crossref] [PubMed]
- Holzapfel GA. Collagen in Arterial Walls: Biomechanical Aspects. Collagen. Boston, MA: Springer US, 2008:285-324.
- Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface 2013;10:20121004. [Crossref] [PubMed]
- Haskett D, Johnson G, Zhou A, et al. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech Model Mechanobiol 2010;9:725-36. [Crossref] [PubMed]
- Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol 2014;13:13-25. [Crossref] [PubMed]
- Prakash A, Adlakha H, Rabideau N, et al. Segmental Aortic Stiffness in Children and Young Adults With Connective Tissue Disorders: Relationships With Age, Aortic Size, Rate of Dilation, and Surgical Root Replacement. Circulation 2015;132:595-602. [Crossref] [PubMed]
- Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res 2013;99:276-83. [Crossref] [PubMed]
- Raaz U, Zöllner AM, Schellinger IN, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015;131:1783-95. [Crossref] [PubMed]
- Goudot G, Mirault T, Bruneval P, et al. Aortic Wall Elastic Properties in Case of Bicuspid Aortic Valve. Front Physiol 2019;10:299. [Crossref] [PubMed]
- Boczar KE, Boodhwani M, Beauchesne L, et al. Aortic Stiffness, Central Blood Pressure, and Pulsatile Arterial Load Predict Future Thoracic Aortic Aneurysm Expansion. Hypertension 2021;77:126-34. [Crossref] [PubMed]
- Chiu P, Lee HP, Dalal AR, et al. Relative strain is a novel predictor of aneurysmal degeneration of the thoracic aorta: An ex vivo mechanical study. JVS Vasc Sci 2021;2:235-46. [Crossref] [PubMed]
- Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int 2010;10:S213-20. [Crossref] [PubMed]
- Barodka VM, Joshi BL, Berkowitz DE, et al. Implications of Vascular Aging. Anesth Analg 2011;112:1048-60. [Crossref] [PubMed]
- van Ooij P, Garcia J, Potters WV, et al. Age-related changes in aortic 3D blood flow velocities and wall shear stress: Implications for the identification of altered hemodynamics in patients with aortic valve disease. J Magn Reson Imaging 2016;43:1239-49. [Crossref] [PubMed]






