Pre- and post-operative mechanical circulatory support in surgical repair of post-acute myocardial infarction mechanical complications
Introduction
Given the advances in pharmacological, catheter-based and surgical reperfusion, the mechanical complications following acute myocardial infarction (AMI) are less frequent but remain catastrophic in ST-segment elevation myocardial infarctions (STEMIs) (1). They may involve the interventricular septum, the ventricular free wall, or the papillary muscles. Although the incidence of mechanical complications is low, the associated mortality rate remains quite high (1). Furthermore, due to lack of an optimal, evidence-based therapeutic strategy and complex decision-making involved in the management of these complications, a multidisciplinary ‘Heart team’ approach is required. With the paucity of data to guide clinical practice, there is significant variability in management of these complications (1). In this review, we discuss the pre- and post-operative role of temporary mechanical circulatory support (MCS) devices in surgical repair of post-AMI mechanical complications.
Ventricular septal rupture (VSR)
Ventricular septal defect or VSR is the most common mechanical complication of transmural AMI with a reported incidence of around 0.3% (2). VSR is typically seen 3–5 days after anterior or inferior infarction with older age, female sex, and delayed reperfusion being the most common risk factors (3). Anterior infarctions are more likely to cause apical defects with inferior/lateral infarctions more likely to cause basal defects at the junction of the septum and posterior wall (3). Regardless of location, VSR causes shunting of oxygenated blood from the left to right ventricle.
The clinical presentation varies from dyspnea to frank circulatory collapse depending on the size of the defect, presence of right ventricular (RV) ischemia or infarction, or RV overload. Despite improving reperfusion strategies and mortality with AMI, the outcome of patients who develop VSR remains poor (3). Initially, classical teaching was immediate surgical repair, however lately, optimal timing of surgical treatment is an ongoing debate and should be discussed amongst the Heart team, including a cardiac surgeon, heart failure cardiologist, interventionalist, and a cardiac intensivist. No clear evidence is available to guide the surgical management of patients who are in cardiogenic shock and a high mortality is associated with all strategies (4). Medical management is tricky as use of inotropes may increase cardiac output and blood pressure but worsens shunt flow, thereby providing no improvement to the hemodynamic profile. Similarly, vasopressors increase blood pressure but worsen shunt flow and decrease systemic perfusion. Use of vasodilators may be precluded by hypotension in these critically ill patients (5). Jeppsson et al. reported a high 30-day mortality rate for VSR (51% for posterior and 30% for anterior VSR), which rose to 68% for posterior VSRs operated upon within 48 hours (6). One of the recommended approaches is to place a percutaneous MCS device prior to a delayed surgical or percutaneous intervention (7).
Pre-operative use of MCS
Different types of MCS devices may have different interactions with the pathophysiology of VSRs, and the ideal MCS configuration should be tailored to the patient to avoid maladaptive changes (8). The intra-aortic balloon pump (IABP) is the most used device that provides mechanical afterload reduction and decreased left-to-right shunt flow, which augments cardiac output (9). This can be utilized routinely, even in hemodynamically stable patients before the onset of RV failure and end-organ damage. Moreover, the rupture sites can expand, and the resulting hemodynamic compromise is rapid and may be fatal (10).
Data suggests that in a substantial group of patients with VSRs, early initiation of veno-arterial extracorporeal membrane oxygenation (V-A ECMO) support may be superior to IABP (11). There are mostly case reports regarding the use of biventricular V-A ECMO support in post-infarct VSR (12,13). ECMO-supported patients should be monitored closely for signs of aortic valve closure, predisposing individuals to aortic root thrombus formation. In addition, patients should be monitored for lack of improvement or even worsening of left filling pressures, which may warrant use of a mechanical left ventricular (LV) venting strategy (5). Extra caution should be exercised when considering LV venting strategies in patients with VSR, due to the potential risk of right-to-left shunting deoxygenated blood and embolization of necrotic LV debris with the use of an Impella device (Abiomed, Danvers, MA) (1). Per these case reports, ECMO support is continued post-operatively to offload both ventricles and reduce risk of patch dehiscence (12,13).
Theoretically, the presence of an interventricular shunt and a large area of necrosis (frequently involving the apex of the heart) may be considered limitations in the use of peripheral ventricular assist devices (pVADs). However, there are small case series and case reports regarding the use of Impella for post-infarct VSR (14-16). Use of Impella Recover 5.0 in cases of cardiogenic shock due to posterior ventricular septal defect seems to be a feasible and safe way to improve hemodynamics while awaiting surgery (14).
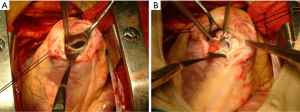
Another pVAD, TandemHeart (LivaNova, London, UK), unloads the failing LV by directing blood from the left atrium to the pump via an inflow cannula that is inserted into the femoral vein and advanced across the interatrial septum into the left atrium. The flow dynamics are similar to surgically implanted axial flow devices that augment end-organ perfusion. In our previously published experience, the TandemHeart pVAD offers additional advantages as it rarely causes hemolysis and poses minimal risk of aspiration of necrotic myocardial debris into the pump (17). Additionally, it does not cause right-to-left shunting since the inflow cannula is placed in the left atrium. Figure 1 show a surgical repair of VSR on a patient supported with TandemHeart. It can also be used in patients with critical aortic stenosis, left ventricular thrombus, and mechanical aortic valves; it can truly be placed percutaneously. This device can provide additional safety during percutaneous VSR closure where circulatory collapse is a possibility (18). Limitations include the need for trans-septal puncture, vascular access-related complications, and the associated risk for cannula malposition or dislodgement.
There are case reports of other surgical VADs including intracorporeal continuous-flow axial or centrifugal pumps used for post-infarct VSR as a bridge to transplantation, bridge to recovery, or destination therapy (8,19,20). They are seldom used for first-line pre-operative MCS and are usually considered in refractory cases who have failed VSR repair as a bridge to transplant. Patients who are not responding to percutaneous mechanical support and are not candidates for surgical repair or percutaneous closure due to size and location of the VSD may be considered for a total artificial heart as a bridge to transplant (21).
Also, percutaneous closure of VSR is a feasible treatment option in carefully selected, high-risk patients and may avoid or delay surgical repair; however, head-to-head comparisons between the two strategies are lacking (22). At the end of the day, the key principle in any intervention post-infarct VSR is to establish adequate circulatory support ensuring end-organ perfusion and allowing the infarcted area and adjacent tissue to obtain some degree of healing fibrotic tissue to ensure suture-line integrity during the repair (23).
Post-operative use of MCS
Case reports have shown removal of MCS devices at different timings; however, no data favors any duration of support post-operatively. Theoretically, post-operative pVAD support has benefits. First, by unloading the LV, it may help gradual ventricular reconditioning following VSR repair. It also helps prevent recurrent septal defect formation early after surgical repair, thereby decreasing mortality related to recurrence of VSR. Post-operative use of TandemHeart reconditions the RV weakened by left-to-right shunting and reconditions the LV due to low resistance caused by VSD (17).
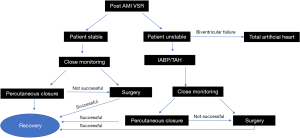
Other alternatives for patients who are ineligible for traditional VSR patch repair include percutaneous closure, use of mechanical support as bridge to advanced heart failure therapies including heart transplant or total artificial heart, and palliative medical therapy. We propose a simplified algorithm in the management of patients with post-infarct VSR depending on clinical condition and other factors (Figure 2).
Mitral regurgitation (MR)
Acute severe MR from papillary muscle rupture (PMR) is characterized by acute pulmonary edema, hemodynamic instability and cardiogenic shock, ultimately leading to multi-organ failure and death. It is a medical, and often surgical emergency. Although incidence has declined in the reperfusion era, the reported in-hospital mortality rate remains high (24). Prompt surgical intervention remains the mainstay of therapy, however studies show that the peri-operative mortality rate of patients undergoing mitral valve surgery for MR ranges from 14.8% to 27% (24). Furthermore, surgical outcomes in patients undergoing surgery with shock physiology are significantly worse, as compared to patients without cardiogenic shock. Medical management and MCS can stabilize patients in the interim (25).
The mitral valve involves two papillary muscles - the anterolateral and posteromedial. Anterolateral PMR is extremely uncommon due to dual arterial blood supply from the left anterior descending artery and the diagonal or marginal branch of the circumflex coronary artery. Posteromedial PMR typically occurs in association with inferior or lateral STEMIs due to single vessel blood supply from the circumflex coronary artery or the right coronary artery, depending on dominance (26,27). PMR can be complete or partial, subsequently influencing the severity of clinical symptoms.
Initial medical care and resuscitation efforts in the cardiac intensive care unit involves respiratory support with non-invasive or invasive mechanical ventilation. However, choosing a vasopressor or an inotropic agent involves careful evaluation of hemodynamics as an increase in either preload or inotropy can increase MR with subsequent worsening of pulmonary edema. Afterload reduction with vasodilators is theoretically ideal but is frequently limited by hypotension in these patients (26).
Pre-operative use of MCS
The pre-operative stabilization of cardiogenic shock with PMR is complex. Such cases warrant a multi-disciplinary team discussion and initiation of temporary MCS. The implantation of an MCS device can stabilize patients and is generally accepted as the standard of care until urgent surgery can be performed safely. IABP is a widely available device that decreases afterload, thereby decreasing the MR. However, it offers minimal cardiac output augmentation (27,28).
The Impella device offers more robust cardiac output augmentation and directly unloads the LV, which improves oxygenation and hemodynamics as it decreases retrograde flow across the mitral valve (29). ECMO use alone may increase afterload and potentially worsen the MR (30). Ventricular distension is commonly seen in these patients. This is important as it increases the myocyte oxygen consumption and myofibrillar disarrangement, especially in the post-operative state. The TandemHeart device can directly unload the left atrium and potentially offer the best hemodynamic effect in patients with MR. Also, its use with an oxygenator (TH-ECMO) can provide prompt hemodynamic stabilization and resolution of hypoxemia to enable early surgery and increase the odds of a favorable outcome (31).
There are no randomized control trials of MCS use in post-MI-MR; only case reports are available. In these cases, the devices were removed intra-operatively or immediately post-operatively.
Free-wall rupture
Post-MI free-wall rupture is an infrequent but life-threatening complication with an incidence ranging from 0.2% to 7.6% (32). Free-wall ruptures involve (I) an abrupt, slit-like tear associated with acute infarcts within 24 hours; (II) erosion of the infarcted myocardium with a sub-acute presentation; or (III) concomitant aneurysm formation with significant thinning of the septum and subsequent rupture associated with older infarcts (3).
The clinical presentation varies from catastrophic cardiogenic shock, electromechanical dissociation and cardiac arrest, to oozing into the pericardium with hemodynamic instability (33).
There should be a high index of suspicion for free-wall rupture in any post-infarction patient with sudden hemodynamic deterioration, warranting an expedited echocardiogram to confirm the diagnosis. Surgery is the only life-saving option in most cases but is associated with >35% inpatient mortality (32,33).
Limited data suggests that delayed MCS has the poorest prognosis, so prompt resuscitation is strongly recommended (34). Reports discuss the use of IABP for circulatory support but it has a very limited role, if any (33). There are reports of emergent ECMO placement in patients with cardiogenic shock related to cardiac tamponade but poor venous return related to tamponade may impede the ECMO flows (32).
Post-operatively, the use of IABP and other MCS devices can reduce intra-cavitary pressure of the LV, increase coronary blood flow, and limit the development of low output state (33). MCS using VADs can wean patients from cardiopulmonary bypass after surgical closure of the LV free-wall rupture and provide LV decompression. However, we need more data to better evaluate the efficacy and safety of mechanical devices in the setting of post-infarction free wall rupture.
Conclusions
Post-infarction mechanical complications are associated with hemodynamic instability and characterized by a peculiar pathophysiology and hemodynamic profile. There is evidence, albeit limited, regarding the benefit of hemodynamic stabilization prior to attempting permanent repair. Achieving hemodynamic stabilization in critical patients delays surgical repair. This delay is associated with improved outcomes, particularly in VSR, and contributes to improved survival post-surgery. Furthermore, each MCS device has a different mechanism, and the ideal MCS configuration should be identified as per patient characteristics and hemodynamic profiling. Given the limited data, we need more advanced, dedicated studies to better clarify the role of MCS as a bridge to definitive surgical repair in these patients.
Acknowledgments
The authors thank Drs. Jessica Moody and Michelle Gehring for editorial support.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Damluji AA, van Diepen S, Katz JN, et al. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021;144:e16-35. [Crossref] [PubMed]
- Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv 2019;12:1825-36. [Crossref] [PubMed]
- Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J 2014;35:2060-8. [Crossref] [PubMed]
- Papalexopoulou N, Young CP, Attia RQ. What is the best timing of surgery in patients with post-infarct ventricular septal rupture? Interact Cardiovasc Thorac Surg 2013;16:193-6. [Crossref] [PubMed]
- Pahuja M, Schrage B, Westermann D, et al. Hemodynamic Effects of Mechanical Circulatory Support Devices in Ventricular Septal Defect. Circ Heart Fail 2019;12:e005981. [Crossref] [PubMed]
- Jeppsson A, Liden H, Johnsson P, et al. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg 2005;27:216-21. [Crossref] [PubMed]
- Arnaoutakis GJ, Zhao Y, George TJ, et al. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg 2012;94:436-43; discussion 443-4. [Crossref] [PubMed]
- Ronco D, Matteucci M, Ravaux JM, et al. Mechanical Circulatory Support as a Bridge to Definitive Treatment in Post-Infarction Ventricular Septal Rupture. JACC Cardiovasc Interv 2021;14:1053-66. [Crossref] [PubMed]
- Singh V, Rodriguez AP, Bhatt P, et al. Ventricular Septal Defect Complicating ST-Elevation Myocardial Infarctions: A Call for Action. Am J Med 2017;130:863.e1-863.e12. [Crossref] [PubMed]
- Thiele H, Lauer B, Hambrecht R, et al. Short- and long-term hemodynamic effects of intra-aortic balloon support in ventricular septal defect complicating acute myocardial infarction. Am J Cardiol 2003;92:450-4. [Crossref] [PubMed]
- Rob D, Špunda R, Lindner J, et al. A rationale for early extracorporeal membrane oxygenation in patients with postinfarction ventricular septal rupture complicated by cardiogenic shock. Eur J Heart Fail 2017;19:97-103. [Crossref] [PubMed]
- Rohn V, Spacek M, Belohlavek J, et al. Cardiogenic shock in patient with posterior postinfarction septal rupture--successful treatment with extracorporeal membrane oxygenation (ECMO) as a ventricular assist device. J Card Surg 2009;24:435-6. [Crossref] [PubMed]
- Neragi-Miandoab S, Michler RE, Goldstein D, et al. Extracorporeal membrane oxygenation as a temporizing approach in a patient with shock, myocardial infarct, and a large ventricle septal defect; successful repair after six days. J Card Surg 2013;28:193-5. [Crossref] [PubMed]
- La Torre MW, Centofanti P, Attisani M, et al. Posterior ventricular septal defect in presence of cardiogenic shock: early implantation of the impella recover LP 5.0 as a bridge to surgery. Tex Heart Inst J 2011;38:42-9. [PubMed]
- Patanè F, Zingarelli E, Sansone F, et al. Acute ventricular septal defect treated with an Impella recovery as a 'bridge therapy' to heart transplantation. Interact Cardiovasc Thorac Surg 2007;6:818-9. [Crossref] [PubMed]
- Via G, Buson S, Tavazzi G, et al. Early cardiac unloading with ImpellaCP™ in acute myocardial infarction with ventricular septal defect. ESC Heart Fail 2020;7:708-13. [Crossref] [PubMed]
- Gregoric ID, Mesar T, Kar B, et al. Percutaneous ventricular assist device and extracorporeal membrane oxygenation support in a patient with postinfarction ventricular septal defect and free wall rupture. Heart Surg Forum 2013;16:E150-1. [Crossref] [PubMed]
- Gregoric ID, Bieniarz MC, Arora H, et al. Percutaneous ventricular assist device support in a patient with a postinfarction ventricular septal defect. Tex Heart Inst J 2008;35:46-9. [PubMed]
- Pitsis AA, Kelpis TG, Visouli AN, et al. Left ventricular assist device as a bridge to surgery in postinfarction ventricular septal defect. J Thorac Cardiovasc Surg 2008;135:951-2. [Crossref] [PubMed]
- Hlaváček D, Pokorný M, Ivák P, et al. Implantation of durable left ventricular assist device in patient with postmyocardial infarction ventricular septal defect. J Card Surg 2021;36:3944-7. [Crossref] [PubMed]
- Gregoric ID. Total artificial heart in patients with post-infarction ventricular septal defect. Ann Cardiothorac Surg 2020;9:116-7. [Crossref] [PubMed]
- Schlotter F, de Waha S, Eitel I, et al. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention 2016;12:94-102. [Crossref] [PubMed]
- Loyolka P, Nascimbene A, Nathan S, et al. Improved outcomes in the treatment of post-myocardial infarction ventricular septal defect with percutaneous TandemHeart left ventricular mechanical circulatory support. Available online: https://digitalcommons.library.tmc.edu/cgi/viewcontent.cgi?article=1011&context=vad
- French JK, Hellkamp AS, Armstrong PW, et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI). Am J Cardiol 2010;105:59-63. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, De Cicco G, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573-82. [Crossref] [PubMed]
- Kutty RS, Jones N, Moorjani N. Mechanical complications of acute myocardial infarction. Cardiol Clin 2013;31:519-31. vii-viii. [Crossref] [PubMed]
- Bhardwaj B, Sidhu G, Balla S, et al. Outcomes and Hospital Utilization in Patients With Papillary Muscle Rupture Associated With Acute Myocardial Infarction. Am J Cardiol 2020;125:1020-5. [Crossref] [PubMed]
- Dekker AL, Reesink KD, van der Veen FH, et al. Intra-aortic balloon pumping in acute mitral regurgitation reduces aortic impedance and regurgitant fraction. Shock 2003;19:334-8. [Crossref] [PubMed]
- Jalil B, El-Kersh K, Frizzell J, et al. Impella percutaneous left ventricular assist device for severe acute ischaemic mitral regurgitation as a bridge to surgery. BMJ Case Rep 2017;2017:bcr-2017-219749. [Crossref] [PubMed]
- Villablanca P, Nona P, Lemor A, et al. Mechanical Circulatory Support in Cardiogenic Shock due to Structural Heart Disease. Interv Cardiol Clin 2021;10:221-34. [Crossref] [PubMed]
- DiVita M, Visveswaran GK, Makam K, et al. Emergent TandemHeart-ECMO for acute severe mitral regurgitation with cardiogenic shock and hypoxaemia: a case series. Eur Heart J Case Rep 2020;4:1-6. [Crossref] [PubMed]
- Formica F, Mariani S, Singh G, et al. Postinfarction left ventricular free wall rupture: a 17-year single-centre experience. Eur J Cardiothorac Surg 2018;53:150-6. [Crossref] [PubMed]
- Matteucci M, Fina D, Jiritano F, et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care 2019;8:379-87. [Crossref] [PubMed]
- Okada K, Yamashita T, Matsumori M, et al. Surgical treatment for rupture of left ventricular free wall after acute myocardial infarction. Interact Cardiovasc Thorac Surg 2005;4:203-6. [Crossref] [PubMed]






