Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection - analysis of the German Registry for Acute Aortic Dissection type A (GERAADA)
Introduction
Acute aortic dissection type A (AADA) remains a life threatening medical condition requiring emergent surgical therapy. Despite improvements in diagnostics, medical therapy and surgical technique, patient mortality and morbidity remains high (1). Standard treatment in the setting of AADA is the replacement of the ascending aorta with resection of the entry site, often in combination with an open distal anastomosis or hemiarch replacement, during a period of circulatory arrest with implementation of adjunct neuroprotective strategies such as cerebral perfusion and hypothermia (2). However, this treatment leaves the downstream aorta untouched and a residual dissection membrane remains in up to 70% of patients treated for AADA (3-7). The risk of progressive dilation with possible need for aortic re-intervention over the long-term remains (8-11). Due to this risk, a more aggressive approach with complete arch replacement and possible stenting of the proximal descending aorta via an antegrade approach has been adopted by a number of clinical institutions worldwide, to better obliterate the false lumen and thus reduce the incidence of late aortic complications (12-14). Other groups, however, have demonstrated an increased risk of mortality and morbidity when extensive surgery involving the aortic arch and the downstream aorta is implemented, thus recommending a more conservative approach to the treatment of AADA patients (15,16).
The German Registry for Acute Aortic Dissection type A (GERAADA) is a web-based registry, initiated by the Working Group for Aortic Surgery and Interventional Vascular Surgery of the German Society for Thoracic and Cardiovascular Surgery. It is presently the largest registry worldwide documenting patients undergoing surgery for AADA (17-19). Analysis of GERAADA gave us the opportunity to compare the surgical outcomes of patients with DeBakey type I dissection treated by total arch replacement and those of hemiarch replacement with respect to early mortality, and onset of new neurological and malperfusion deficit.
Methods
All patients with DeBakey type I aortic dissections enrolled in GERAADA between July 2006 and June 2010 were identified. The dissection entry site was reduced to the ascending aorta. Patients with an entry/re-entry of the aortic arch warranting treatment of the arch during primary surgery were excluded from the data analysis. The patients were then classified into two groups: Group A had conventional treatment with replacement of the ascending aorta and hemiarch replacement/open distal anastomosis. Group B had extensive surgery with complete arch replacement, possibly in combination with an elephant trunk/frozen elephant trunk treatment of the descending aorta.
Data was acquired by use of a standard online questionnaire developed by the GERAADA principal investigator. This included patient demographics, pre- and intraoperative status, postoperative complications, early results, and date of death. Data forms were delivered to the registry on the German Society for Thoracic and Cardiovascular Surgery (GSTCVS) homepage.
Statistics were summarised as frequencies and percentages for categorical variables and as mean and standard deviation for continuous variables. The t-test or Mann-Whitney U test for continuous variables and the chi-squared test or Fischer’s exact test for categorical variables were used as appropriate. Influence of risk factors on 30-day mortality, new neurological deficit and new malperfusion deficit were analysed using logistic regression analysis.
Results
Between July 2006 and June 2010, 50 centres participating in GERAADA reported on a total of 2,137 patients. 658 patients were identified with DeBakey type I aortic dissection and the entry site limited to the ascending aorta. 518 patients were classified in Group A. 140 patients were treated in Group B with total arch replacement, possibly with adjunct therapy of the descending aorta. 172 patients presented with a pre-existing neurological deficit (26.1%; Group A 24.7% vs. Group B 31.4%) and 261 patients presented with a pre-existing malperfusion deficit (39.7%; Group A 38.2% vs. Group B 46.4%).
The operative procedure was significantly shorter with hemiarch replacement (318.1±104.4 mins) than with total arch replacement (390.4±137.3 mins, P<0.001) and the mean duration of circulatory arrest was shorter for Group A than for Group B (24.3±14.4 vs. 44.8±29.7 mins, P<0.001). Postoperative outcomes showed a higher rate of rethoracotomy for Group B (18.5% vs. 28.6%, P=0.013) and a higher rate of bleeding (22.3% vs. 35.7%, P=0.002), defined as excessive bleeding >1,000 mL over 24 hours. Patients in Group B stayed longer in ICU (8.1±11.3 vs. 10.3±13.8 days, P<0.001) and in hospital (16.3± 14.6 vs. 17.1±16.1 days, P<0.001). Further details are presented in Table 1.
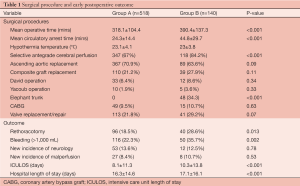
Full table
Overall mortality was 133 patients (20.2%), with a tendency for lower rate of mortality in Group A (18.7%) than in Group B (25.7%), however not reaching a statistically significant difference (P=0.07).
The onset of new neurological deficit showed no difference between the two groups (13.6% vs. 12.5%, P=0.78). The presence of preoperative neurological deficit resulted in no difference concerning the postoperative mortality (32.8% vs. 29.5%, P=0.69). However, if patients underwent surgery without a pre-existing neurological deficit, Group A demonstrated a lower risk of mortality than Group B with the more extensive procedure (14.1% vs. 24%, P=0.02).
No difference was observed between the two groups regarding onset of new malperfusion (8.4% vs. 10.7%, P=0.53). Postoperative mortality in patients with preoperative malperfusion was similar (25.5% vs. 33.8%, P=0.19); in the absence of preoperative malperfusion there was no significant difference (14.6% vs. 18.7%, P=0.37)
Logistic regression analysis of clinical presentation and surgery on 30-day mortality showed age (P=0.0072), preoperative resuscitation (P=0.041), length of cerebral perfusion (P=0.0122) and length of circulatory arrest (P=0.041) to be significant risk factors for early postoperative mortality.
Discussion
Aggressive and extensive surgery with total arch replacement and adjunct treatment of the descending aorta has been described to decrease the prevalence of the patent false lumen with favourable long-term outcomes by maximising the resection and treatment of re-entry points in AADA patients (12,20-23). However, this aggressive approach is performed at the cost of a more invasive operation with longer periods of myocardial ischemia and circulatory arrest. Direct myocardial and cerebral injuries as well as organ dysfunction can be associated with the more complex surgical procedure (2,15-16). Furthermore, other study groups suggest the residually dissected aorta does not decrease late survival with low risk of aneurysmal change and reoperation (24). Dobrilovic and Elefteriades demonstrated a need for reoperation in only 2% of patients for dilation of the descending aorta with a growth rate of only 0.28 cm per year (25). Recently Kim and colleagues described a poorer survival and neurological outcome for patients with total arch replacement, with the rate of reoperation not affected by the type of surgery for AADA, and reoperations being performed without significant mortality or morbidity (26).
Use of GERAADA, the largest worldwide registry for the treatment of AADA, allowed us to analyze a large patient cohort, focusing on the influence of the operative strategy chosen for the aortic arch and its influence on mortality, as well as the onset of new neurological and malperfusion deficit.
Postoperative mortality was 20.2% for DeBakey type I dissection patients in GERAADA, with a tendency for lower postoperative mortality for hemiarch replacement compared to total arch replacement (18.7% vs. 25.7%) failing to be statistically significant. The higher rate of rethoracotomy (P=0.013) and excessive bleeding (more than 1,000 mL/d) (P=0.002) demonstrate the postoperative complications caused by the coagulopathic disorders induced by the prolonged period of circulatory arrest and are factors influencing ICU and overall hospital stay. The onset of new neurological deficit were similar between the hemiarch and the total arch group (13.6% vs. 12.5%, P=0.78), unlike the findings of Kim et al. who found more frequent neurological dysfunction in patients with total arch replacement (26). Overall onset of neurological dysfunction is similar to other reports, with 13.4% after surgery.
End-organ malperfusion and ischemia develop in 16-30% of patients suffering from AADA (27-31), and patients with a pre-existing malperfusion pathology experience postoperative mortality rate of up to 89% (30). In the present analysis, 39.7% of patients presented with a malperfusion deficit, including coronary, spinal, visceral, or peripheral limb ischemia. However, the onset of new malperfusion deficit after surgical treatment showed no difference between the two groups (P=0.53). The prognosis of patients with preoperative malperfusion still remains poor and prolonged periods of cardiopulmonary bypass and large volume blood resuscitation may contribute to a large capillary leak and end organ damage (27).
In view of the data analysed from GERAADA, it may be concluded that more extensive treatment with total arch replacement and possibly adjunct therapy of the descending aorta can be performed in the setting of the AADA at an acceptable operative risk comparable to the standard treatment with replacement of the ascending aorta. Immediate postoperative complications are higher; however 30-day mortality and the onset of new neurological and malperfusion deficit show no significant difference. In the absence of pre-existing neurological deficits, subgroup analysis demonstrates a higher mortality for patients treated with total arch replacement. Long-term results are presently not obtained by the registry data, and are clearly necessary to justify the necessity of possible aortic re-intervention for patients treated for AADA by the differing surgical approaches. Modifications of GERAADA will address this issue with follow-up data included for a more thorough analysis of AADA patients (32).
Acknowledgements
Centers participating in GERAADA: Herzzentrum Leipzig, Klinik für Herzchirurgie, Leipzig, Germany; Universitätsklinikum Frankfurt, Abteilung für Thorax-, Herz- und Thorakale Gefäßchirurgie, Frankfurt am Main, Germany; Universitäres Herz- und Kreislaufzentrum Freiburg - Bad Krozingen, Abteilung für Herz- und Gefäßchirurgie, Freiburg, Germany; Universitätsklinikum Heidelberg, Abteilung für Herzchirurgie, Heidelberg, Germany; Klinikum Augsburg, Klinik für Herz- und Thoraxchirurgie, Augsburg, Germany; Universitätsklinikum Tübingen, Klinik für Thorax-, Herz- und Gefäßchirurgie, Tübingen, Germany; Klinikum der Ludwig-Maximilians-Universität München-Großhadern, Herzchirurgische Klinik und Poliklinik, München, Germany; Universitäres Herzzentrum Hamburg, Klinik und Poliklinik für Herz- und Gefäßchirurgie, Hamburg, Germany; Universitätsmedizin Mainz, Klinik für Herz-, Thorax- und Gefäßchirurgie, Mainz, Germany; Städtisches Klinikum Braunschweig, Klinik für Herz-, Thorax- und Gefässchirurgie, Braunschweig, Germany; Klinikum Oldenburg, Klinik für Herzchirurgie, Oldenburg, Germany; Inselspital Bern, Universitätsklinik für Herz- und Gefässchirurgie, Bern, Switzerland; Herz- und Gefäß-Klinik Bad Neustadt, Abteilung für Kardiochirurgie, Bad Neustadt, Germany; Westdeutsches Herzzentrum Essen, Klinik für Thorax- und kardiovaskuläre Chirurgie, Essen, Germany; Allgemeines Krankenhaus - Universitätskliniken Wien, Abteilung für Herz- und Thoraxchirurgie, Wien, Austria; Universitätsklinikum Ulm, Klinik für Herzchirurgie, Ulm, Germany; Schüchtermann-Klinik Bad Rothenfelde, Abteilung für Herzchirurgie, Bad Rothenfelde, Germany; Herzzentrum Dresden, Klinik für Kardiochirurgie, Dresden, Germany; Universitätsklinikum Schleswig-Holstein Campus Lübeck, Klinik für Herzchirurgie, Lübeck, Germany; Universitätsklinikum Bonn, Klinik und Poliklinik für Herzchirurgie, Bonn, Germany; Kerckhoff-Klinik, Abteilung für Herz- und Thoraxchirurgie, Bad Nauheim, Germany; Klinikum Nürnberg, Klinik für Herzchirurgie, Nürnberg, Germany; Universitätsklinikum Würzburg, Klinik und Poliklinik für Thorax-, Herz- und Thorakale Gefäßchirurgie, Würzburg, Germanyl Herzzentrum Duisburg, Klinik für Thorax- und Kardiovaskularchirurgie, Duisburg, Germany; Universitätsklinikum des Saarlandes Homburg, Klinik für Thorax- und Herz-Gefäßchirurgie, Homburg, Germany; Klinikum Kassel, Klinik für Herz-, Thorax- und Gefäßchirurgie, Kassel, Germany; Universitätsklinikum Münster, Klinik und Poliklinik für Thorax-, Herz- u. Gefäßchirurgie, Münster, Germany; Klinikum Passau, Klinik für Herzchirurgie, Passau, Germany; Albertinen-Krankenhaus Hamburg, Abteilung für Kardiochirurgie, Hamburg, Germany; Herz- und Diabeteszentrum Nordrhein-Westfalen, Abteilung für Thorax- und Kardiovaskularchirurgie, Bad Oeynhausen, Germany; Universitätsklinikum Schleswig-Holstein Campus Kiel, Klinik für Herz- und Gefäßchirurgie, Kiel, Germany; Universitätsklinikum Aachen, Klinik für Thorax-, Herz- und Gefäßchirurgie, Aachen, Germany; Herzzentrum Lahr/Baden, Lahr, Germany; Klinik für Herzchirurgie Karlsruhe, Karlsruhe, Germany; Bundeswehrzentralkrankenhaus Koblenz, Abteilung für Herz- und Gefäßchirurgie, Koblenz, Germany; Schön Klinik Vogtareuth, Klinik für Herzchirurgie, Vogtareuth, Germany; Herz- und Gefäßzentrum Bad Bevensen, Klinik für Herz-Thorax-Chirurgie, Bad Bevensen, Germany; Universitätsklinikum Rostock, Klinik und Poliklinik für Herzchirurgie, Rostock, Germany; Westpfalz-Klinikum Kaiserslautern, Thorax-, Herz- und Gefäßchirurgische Klinik, Kaiserslautern, Germany; Robert-Bosch-Krankenhaus Stuttgart, Klinik für Herz- und Gefäßchirurgie, Stuttgart, Germany; Klinikum Fulda, Klinik für Herz- und Thoraxchirurgie, Fulda, Germany; HELIOS Klinikum Wuppertal, Klinik für Herzchirurgie, Herzzentrum, Wuppertal, Germany; Universitätsklinikum Jena, Klinik für Herz- und Thoraxchirurgie, Jena, Germany; Zentralklinik Bad Berka, Klinik für Kardiochirurgie, Bad Berka, Germany; Herzzentrum des Universitätsklinikums Köln, Klinik für Herz- und Thoraxchirurgie, Köln, Germany; MediClin Herzzentrum Coswig, Klinik für Herz- und Gefäßchirurgie, Coswig, Germany; Medizinische Hochschule Hannover, Klinik für Herz-, Thorax-, Transplantations- und Gefäßchirurgie, Hannover, Germany; Sana Herzchirurgische Klinik Stuttgart, Stuttgart, Germany; Martin-Luther-Universität Halle-Wittenberg, Universitätsklinik und Poliklinik für Herz- und Thoraxchirurgie, Halle, Germany; Klinikum Links der Weser Bremen, Klinik für Thorax-, Herz- und Gefäßchirurgie, Bremen, Germany.
Disclosure: The authors declare no conflict of interest.
References
- Ehrlich MP, Ergin MA, McCullough JN, et al. Results of immediate surgical treatment of all acute type A dissections. Circulation 2000;102:III248-52.
- Westaby S, Saito S, Katsumata T. Acute type A dissection: conservative methods provide consistently low mortality. Ann Thorac Surg 2002;73:707-13.
- Ergin MA, Phillips RA, Galla JD, et al. Significance of distal false lumen after type A dissection repair. Ann Thorac Surg 1994;57:820-4; discussion 825.
- Geirsson A, Bavaria JE, Swarr D, et al. Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 2007;84:1955-64; discussion 1955-64.
- Bachet J, Goudot B, Dreyfus GD, et al. Surgery for acute type A aortic dissection: the Hopital Foch experience [1977-1998]. Ann Thorac Surg 1999;67:2006-9; discussion 2014-9.
- Moore NR, Parry AJ, Trottman-Dickenson B, et al. Fate of the native aorta after repair of acute type A dissection: a magnetic resonance imaging study. Heart 1996;75:62-6.
- Heinemann M, Laas J, Karck M, et al. Thoracic aortic aneurysms after acute type A aortic dissection: necessity for follow-up. Ann Thorac Surg 1990;49:580-4.
- Yeh CH, Chen MC, Wu YC, et al. Risk factors for descending aortic aneurysm formation in medium-term follow-up of patients with type A aortic dissection. Chest 2003;124:989-95.
- Bachet J, Teodori G, Goudot B, et al. Replacement of the transverse aortic arch during emergency operations for type A acute aortic dissection. Report of 26 cases. J Thorac Cardiovasc Surg 1988;96:878-86.
- Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995;92:II113-21.
- Kazui T, Kimura N, Yamada O, et al. Total arch graft replacement in patients with acute type A aortic dissection. Ann Thorac Surg 1994;58:1462-8.
- Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg 2000;119:558-65.
- Sun LZ, Qi RD, Chang Q, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: experience with 107 patients. J Thorac Cardiovasc Surg 2009;138:1358-62.
- Uchida N, Shibamura H, Katayama A, et al. Operative strategy for acute type a aortic dissection: ascending aortic or hemiarch versus total arch replacement with frozen elephant trunk. Ann Thorac Surg 2009;87:773-7.
- Crawford ES, Kirklin JW, Naftel DC, et al. Surgery for acute dissection of ascending aorta. Should the arch be included? J Thorac Cardiovasc Surg 1992;104:46-59.
- Ohtsubo S, Itoh T, Takarabe K, et al. Surgical results of hemiarch replacement for acute type A dissection. Ann Thorac Surg 2002;74:S1853-6; discussion S1857-63.
- Weigang E, Conzelmann LO, Kallenbach K, et al. German registry for acute aortic dissection type A (GERAADA)--lessons learned from the registry. Thorac Cardiovasc Surg 2010;58:154-8.
- Krüger T, Conzelmann LO, Bonser RS, et al. Acute aortic dissection type A. Br J Surg 2012;99:1331-44.
- Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43.
- Immer FF, Krähenbühl E, Hagen U, et al. Large area of the false lumen favors secondary dilatation of the aorta after acute type A aortic dissection. Circulation 2005;112:I249-52.
- Hirotani T, Nakamichi T, Munakata M, et al. Routine extended graft replacement for an acute type A aortic dissection and the patency of the residual false channel. Ann Thorac Surg 2003;76:1957-61.
- Takahara Y, Sudo Y, Mogi K, et al. Total aortic arch grafting for acute type A dissection: analysis of residual false lumen. Ann Thorac Surg 2002;73:450-4.
- Urbanski PP, Siebel A, Zacher M, et al. Is extended aortic replacement in acute type A dissection justifiable? Ann Thorac Surg 2003;75:525-9.
- Sabik JF, Lytle BW, Blackstone EH, et al. Long-term effectiveness of operations for ascending aortic dissections. J Thorac Cardiovasc Surg 2000;119:946-62.
- Dobrilovic N, Elefteriades JA. Stenting the descending aorta during repair of type A dissection: technology looking for an application? J Thorac Cardiovasc Surg 2006;131:777-8.
- Kim JB, Chung CH, Moon DH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg 2011;40:881-7.
- Girardi LN, Krieger KH, Lee LY, et al. Management strategies for type A dissection complicated by peripheral vascular malperfusion. Ann Thorac Surg 2004;77:1309-14; discussion 1314.
- Borst HG, Laas J, Heinemann M. Type A aortic dissection: diagnosis and management of malperfusion phenomena. Semin Thorac Cardiovasc Surg 1991;3:238-41.
- Fann JI, Sarris GE, Mitchell RS, et al. Treatment of patients with aortic dissection presenting with peripheral vascular complications. Ann Surg 1990;212:705-13.
- Aebert H, Cornelius T, Ehr T, et al. Expression of immediate early genes after cardioplegic arrest and reperfusion. Ann Thorac Surg 1997;63:1669-75.
- Lauterbach SR, Cambria RP, Brewster DC, et al. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg 2001;33:1185-92.
- Weigang E, Görgen C, Kallenbach K, et al. German Registry for Acute Aortic Dissection Type A (GERAADA)--new software design, parameters and their definitions. Thorac Cardiovasc Surg 2011;59:69-77.





