Thoracic endovascular repair of chronic type B aortic dissection: a systematic review
Abstract
Background: At present, the optimal management strategy for chronic type B aortic dissection (CTBAD) remains unknown, as equipoise remains regarding medical management versus endovascular treatment versus open surgery. However, the results over recent years of thoracic endovascular aortic repair (TEVAR) in CTBAD appear promising. The aim of this systematic review was to provide a comprehensive analysis of the available data reporting outcomes and survival rates for TEVAR in CTBAD.
Methods: Electronic searches of six databases were performed from inception to April 2021. All studies reporting outcomes, specifically 30-day mortality rates, for endovascular repair of CTBAD were identified. Relevant data were extracted, and a random-effects meta-analysis of proportions or means was performed to aggregate the data. Survival data were pooled using data derived from original Kaplan-Meier curves, which allows reconstruction of individual patient data.
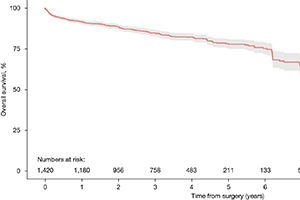
Results: Forty-eight studies with 2,641 patients were identified. Early (<30 days) all-cause and aortic-related mortality rates were low at 1.6% and 0.5%, respectively. Incidence of retrograde type A dissection in the post-operative period was only 1.4%. There were also low rates of cerebrovascular accidents and spinal cord injury (1.1% and 0.9%, respectively). Late follow-up all-cause mortality was 8.0%, however, late aortic-related mortality was only 2.4%. Reintervention rates were 10.1% for endovascular and 6.7% for surgical reintervention. Pooled rates of overall survival at 1-, 3-, 5- and 10-year were 91.5%, 84.7%, 77.7% and 56.3%, respectively.
Conclusions: The significant heterogeneity in the available evidence and absence of consensus reporting standards are important considerations and concern when interpreting the data. Evaluation of the evidence suggests that TEVAR for CTBAD is a safe procedure with low rates of complications. However, the optimal treatment strategy for CTBAD remains debatable and requires further research. Evidence from high-quality registries and clinical trials are required to address these challenges.
Cover