Is tri-port totally thoracoscopic surgery for mitral valve replacement a feasible approach?
Introduction
The increasing adoption of minimally invasive surgical techniques has led to a growing number of surgeons exploring its application in cardiac surgery. In the past decade, minimally invasive surgical techniques in cardiology have undergone many innovations including small incisions in the superior sternum, right anterolateral thoracotomy with or without thoracoscopic assistance and totally thoracoscopic cardiac surgery (TCS) (1-3). At present, TCS can be generally designated into two broad categories of either surgery with thoracoscopic assistance or a totally TCS. Thoracoscopic assisted cardiac surgery is a commonly used technique which is performed under direct vision via a relatively small incision with thoracoscopic assistance. In comparison, totally TCS is a variant of robotic-assisted minimally invasive surgery and has been proved to be safe and feasible for cardiac operations (4-6).
TCS is deemed more appealing with reduced surgical trauma, pain, and recovery time. Despite the advantages of this technique and patient’s preference, its prevalence remains low because of the complexity of robotic techniques, the cost of robotic disposables, and its relatively steep learning curve. Consequently, cardiac surgeons are looking for perfect totally TSC without the necessity of robotic assistance. In response, the present state-of-the-art surgical techniques focus on port-access TCS techniques without robotic assistance to attain the desired attributes of a totally endoscopic surgery. Professor Ma and his team have explored and extended this technique by utilizing a triplet of ports, which we called Ma’s tri-port thoracoscopic cardiac surgery technique (abbreviated as MTCST). MTCST enables the heart operation to be performed through three small ports in the right chest wall without assistance of the Da Vinci robotic system. This technique successfully overcomes the drawbacks of small-incision-based cardiac surgery with its limited field of vision, procedural challenges, considerable trauma, and postoperative pain, while circumventing the shortcomings of conventional sternotomy cardiac surgery (7,8).
Due to the multiple limitations of the robotic system, robotic–assisted cardiac surgery is also not suitable for routine application in developing countries. Furthermore, totally TCS without robot assistance is mastered merely by limited surgeons worldwide, which has led to very few reports about the experiences of totally TCS for mitral valve replacement (MVR). MTCST has been applied in repairing both atrial septal defects (ASD) and ventricular septal defects (VSD), which offered us with rich experiences on MTCST (7,9). Herein, we will demonstrate the application of MTCST for patients undergoing MVR and establish the safety and feasibility of this technique based on a relatively large patient cohort. In addition, the in-hospital results and early outcomes of the patients undergoing the procedure are compared with those undergoing conventional median sternotomy to confirm the reliability of MTCST.
Methods
Patients selection
This study was approved by the Institutional Review Board of Qilu Hospital. Written informed consent and operation-related permission were obtained from each patient. Between June 1, 2013 and September 30, 2019, 490 patients underwent single MVR or concomitant with tricuspid annuloplasty, and were separated into the MTCST group (the MT group, n=267) and conventional median sternotomy group (the MS group, n=223). The inclusion criteria were as follows: (I) echocardiographic examination showing rheumatic mitral valve stenosis with or without regurgitation; (II) absence of atrial fibrillation and left ventricular ejection factor greater than 30%; (III) absence of other heart disease or previous cardiac surgery; and (IV) no other cardiovascular disease or chronic illness. Patients in the MT group were also required to be: (I) less than 100 kg or the diaphragm was not at a very high level, otherwise the chest cavity was reduced or the operation space was limited; (II) the peripheral vessels were normal and there was no risk of peripheral cardiopulmonary bypass (CPB) establishment; (III) there was no history of right thoracic surgery or pleural adhesion and no infectious effusions in the chest. All the operations in MT group were performed by Dr. Ma and the operations in MS group were performed by other surgeons in our team. The primary outcomes of interest were the in-hospital mortality and re-operation. The secondary outcomes were operation time, CPB time, blood loss, ventilation time, ICU stay, hospitalization duration and total costs during hospitalization.
Anesthesia and establishment of cardiopulmonary bypass (CPB)
After induction of venous anesthesia, a transesophageal echocardiographic (TEE) probe was inserted. The respiration rate was maintained 20–30 times/min and the arterial oxygen saturation was kept over 97%. Peripheral CPB was established through the right femoral artery for perfusion and vein for drainage. The cannula was chosen according to the patients’ weight, body surface area, vascular diameter, and the estimated pump flow. Usually 18–24 F arterial cannula and 16–22 F Carpentier double-lumen catheter for venous drainage (Medtronic Inc or Kangxin. Com, China) were used. The arterial cannula was inserted with the guide of guidewire and the venous cannula site was confirmed by the monitor of TEE. The hematocrit of all the patients was maintained at 22–24% during CPB and 28–30% at the end of operation. If the hematocrit was lower than the mentioned levels, transfusion of red blood cells (RBC) was needed.
Surgical techniques
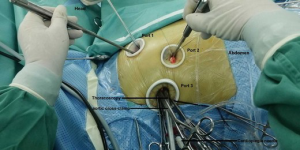
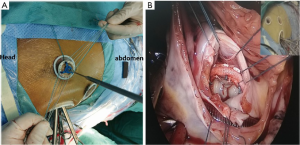
The patients were placed in a supine position with their right side elevated 15–20º and external defibrillation electrodes attached to their skin. Three small incisions (2.5–3 cm each) on the right chest were made according to the methods as previously described (7,9). Generally, the endoscopic port (port 3) was made first for the thoracoscope insertion to explore the right thoracic cavity. Port 3 was located at the fourth intercostal space of the anterior axillary line. Then, the left-hand port (port 1) was made at the right midclavicular line of the second or third intercostal space and the right-hand port (port 2) was located at the fifth or sixth intercostal space between the midclavicular line and the anterior axillary line (Figure 1). Tissue retractors (Hangtian Kadi, Beijing, China) were used to keep the incisions open and for the entry of the long-shafted instruments (Scanlan International, St Paul, Minn) or the thoracoscope (Hopking 30º 26003 BA Karl Storz GmbH & Co., Tuttlingen, Germany or Olympus Corporation, Tokyo, Japan). After the establishment of total CPB, the patients were cooled to 34 °C, and an aortic cross-clamp was positioned on the ascending aorta through port 3. Cardiac arrest was achieved by the delivery of cardioplegia solution through a specially-lengthened needle which was inserted into the aortic root. The lowest nasopharyngeal temperature was maintained between 31–32 °C during the operation. The malformed mitral valve was removed through the previous incision through the atrial septum. First, double needles with a pad were sutured interruptedly into the mitral annulus under the view of the thoracoscope. Next, a mechanical or bioprosthetic valve was sutured in sequence with the needles outside the chest (Figure 2A). Then, the prosthetic valve and the sutures were placed into the heart through port 1, and every suture was tied individually with the use of a knotting instrument (Figure 2B). The incisions of the atrial septum and right atrial wall were closed in sequence and the tricuspid valve was routinely tested by filling with a volume of water before the closure of the right atrium. Tricuspid annuloplasty was performed using Edwards MC3 (Edwards Lifesciences Corporation, USA) if moderate regurgitation was observed. After fully de-airing, the aortic cross-clamp was released and cardiac arrest was terminated. Once the analysis of TEE was satisfactory, patients were weaned from CPB and protamine was administered for heparin neutralization. A 24F drainage tube was put into the right pleural space through port 2 for at least two days and the three small incisions were closed.
After the operation, all patients were transferred to the cardiac intensive care unit (ICU) with intubation. Bedside chest radiography was performed at the second postoperative day or whenever needed to exclude pulmonary complications such as atelectasis, pneumothorax, and pleural effusion. Computerized tomography was used for further diagnosis of any pulmonary complications when needed.
Follow-up
Echocardiography data, a chest X-ray and electrocardiogram were obtained before discharge, at the postoperative 3-month checkup and at the regular check in clinic thereafter. A questionnaire of satisfaction grading the quality of medical treatment was completed by the patients. The questionnaire included the degree of satisfaction with both clinical results and cosmetic results of the surgery. The Medical Treatment Satisfaction Score were graded from 5 (very satisfactory) to 0 (not at all satisfactory) for evaluating both the clinical and the cosmetic results of the surgery. A score of 5 was judged as very satisfactory, 4 as satisfactory, 3 as fine, and 2 to 0 as a little unsatisfactory, unsatisfactory, and very unsatisfactory, respectively. Patients were also verbally questioned about how fast they had regained expected activity levels and the duration of time for them to return to their previous occupations, postoperatively.
Definitions
The drainage volume was defined as the drainage within the first 24 hours following the operation. After extubation and with the patients awake, subjective pain degrees were evaluated using visual analogue scale (VAS) which ranged from 0 (no pain) to 10 (extremely painful). Patients chose the rating scale of pain degree corresponding to their own sensations. Both the patient and the intensivist were blinded for the purpose of VAS. Medical treatment was performed based on the VAS. Ibuprofen and flurbiprofen were administrated for moderate and severe pain, respectively. Postoperative mortality was defined as death occurring from the time of arrival to ICU to their time of discharge. Mortality during follow-up was defined as death occurring from the time of discharge to the first postoperative three months. Hospitalization costs were defined as the total costs of medical treatment which included preoperative test costs, operating room costs, equipment costs, implantation materials costs, the ICU and hospital accommodation costs.
Statistical analysis
SPSS software version 19.0 was used for statistical analysis. Quantitative data with a normal distribution were represented by mean ± standard deviations, while the data with a non-normal distribution (including ICU stay and hospital stay, etc.) were described by median and interquartile range (IQR; P25 and P75). We used standard t-test to compare continuous variables and chi-square analysis to analyze categorical variables. Non-parametric test was used for non-parametric continuous value comparisons. Paired t-test was used for the comparison of preoperative and postoperative data in the same group. Tests with a P value <0.05 were considered statistically significant.
Results
Preoperative characteristics
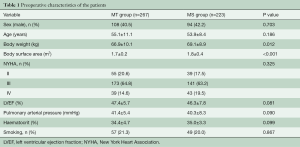
The preoperative characteristics of the patients are shown in Table 1. Body weight (66.9±10.1 vs. 69.1±8.9 kg, P=0.012) and body surface area (1.7±0.2 vs. 1.8±0.4 m2, P<0.001) in the MT group were lower than that in the MS group. Other indexes including demographics, New York Heart Association (NYHA) cardiac function class, left ventricular ejection fraction and pulmonary arterial pressure in the two groups were almost similar (Table 1). The preoperative characteristics were comparable between the two groups.
Full table
Intraoperative and postoperative profile
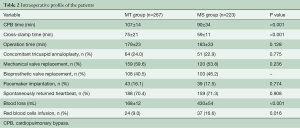
There was no intraoperative death and there were no patients in the MT group whose surgical approach was changed to a median sternotomy. The CPB time and cross-clamp time in the MT group were both longer than in the MS group (107±14 vs. 90±34 min, P<0.001 and 75±21 vs. 59±11 min, P<0.001, respectively). There was no significant difference in the operation time between the two groups (Table 2). The concomitant procedure for tricuspid annuloplasty in the MT group was the same as in the MS group. No difference was observed in the rate of pacemaker implantation and the spontaneous return of heartbeat between the two groups. Compared with the MS group, intraoperative blood loss was significantly less (168±12 vs. 430±54 mL, P<0.001) and the RBC transfusion rate (9.0% vs. 16.6%, P=0.016) was lower in the MT group.
Full table
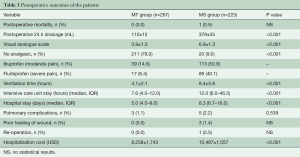
There were no in-hospital deaths in the MT group but one patient in the MS group died of acute heart failure at postoperative Day 2 (Table 3). Drainage volume in the MT group was evidently decreased than that in the MS group (110±12 vs. 379±35 mL, P<0.001). Postoperative VAS in the MT group was lower than the MS group (3.9±1.0 vs. 6.9±1.3, P<0.001). The rates of analgesic administration for the moderate and severe pain were reduced compared to that in the MS group (14.6% vs. 50.9% and 6.4% vs. 40.1%, P<0.001). Ventilation time (4.1±2.1 vs. 8.4±5.8 h, P<0.001), ICU stay [7.6 h (IQR 4.0–12.0) vs. 12.0 h (IQR 6.0–46.5), P<0.001) and the postoperative hospital duration [5.0 d (IQR 4.0–9.0) vs. 8.3 d (IQR 6.7–16.0), P<0.001] in the MT group was shorter than that in the MS group (Table 3). In the MS group, one patient underwent re-operation due to the excessive drainage and three patients developed poor healing of the incision while no undesirable healing and re-operation occurred in the MT group. Pulmonary complications included atelectasis (three in the MT group and two in the MS group) and pleural effusion (three in the MS group). The mean hospital cost (USD) in the MT group was significantly decreased than that in the MS group (8,258±1,743 vs. 10,487±1,557, P<0.001).
Full table
Follow-up
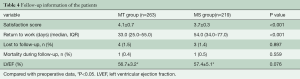
Patients were routinely followed up in clinic during the first 3 months after operation. One patient in the MT group died as a result of a traffic accident and one patient in the MS group died from cerebral embolism due to insufficient anticoagulation after discharge. Another three patients in the MT group and two patients in the MS group were unable to come to the outpatient clinic in person due to the relocation of their children or other reasons. These patients’ follow-up information was not completed and excluded from the study. The echocardiographic examination of the patients showed no paravalvular leak or any other abnormality of the mitral valve, however two patients in the MS group developed moderate tricuspid regurgitation. New-onset atrial fibrillation occurred in one patient of the MT group and three patients of the MS group. There was no significant difference in the left ventricular ejection faction (LVEF) between the two groups at their postoperative 3-month follow-up. There was a distinct increase in the LVEF in both the MT and MS groups as compared to their respective preoperative levels, with all contacted patients having no limitations to their physical activity levels postoperatively (Table 4). Patients in the MT group returned to their occupations (normal pre-operative jobs) much earlier than that in the MS group [33.0 d (IQR 25.0–55.0) vs. 54.0 d (IQR 34.0–77.0), P<0.001]. Most of the patients in the MT group were satisfied with both the surgical outcomes and cosmetic results of the procedure. The mean Medical Treatment Satisfaction Score in the MT group was higher than that in the MS group (4.1±0.7 vs. 3.7±0.3, P<0.001).
Full table
Discussion
In this study, based on the endoscopic techniques reported previously (10,11), we implemented and studied the application of MTCST without the aid of the robotic Da Vinci surgical system in patients undergoing MVR in order to establish the safety and feasibility of this technique. The results demonstrated: (I) MVR by MTCST was a safe and feasible surgical option; (II) compared with conventional sternotomy for MVR, MTCST reduced the total operative blood loss, need for blood transfusion, postoperative pain levels, ICU duration, hospital stay, and the hospitalization cost; (III) compared with median sternotomy, MTCST was a suitable and desirable approach for MVR surgery.
Mitral valve surgery, in the setting of prior cardiac surgery, remained a continuing challenge in its ability to offer patients a safe and effective valve repair or replacement (11). The right anterior thoracotomy had been shown as effective in avoiding a median sternotomy as well as potential complications associated with the sternotomy (1,12). This approach enabled both direct exposure and excellent visibility of the entire left (and right) atrium. The combination of MTCST and modern bypass techniques allowed mitral valve operations to be performed through three small ports in the right chest. Moreover, MTCST had also previously displayed its feasibility and efficacy in cardiac operations for congenital heart disease including ASD and VSD closures with overcoming the limitations of the robotic technology (7-9). The present study demonstrates that MVR with MTCST achieves a minimal degree of invasiveness on the premise of attaining the same safety and efficacy as conventional and right thoracotomy cardiac surgery. In fact, MTCST provided surgeons with a relatively wide and magnified field under the view of the thoracoscope allowing the operation to be performed effectively where it was previously only conducted with open exposure. Therefore, this approach also belongs to the category of microsurgery to some extent because the inside structure of heart was observed more clearly by the surgeons. As far as this respect concerned, MTCST for MVR was safer than the conventional sternotomy.
In our study, both the CPB time and cross-clamp time in the MT group were longer than that in the MS group. This result might be explained by every delicate manipulation under the vision of the thoracoscope. It seemed that the CPB time of the MT group was similar to other reports on video-assisted minimally invasive surgery for MVR (10,13). Even though the CPB and cross-clamp time in the MT group were longer than that in the MS group, there was no significant difference on the whole operation time compared with that in the MS group. This result might be attributed to the short time of small incision sutures and the avoidance of sternal closure in the MT group. In addition, the lower level of blood loss and the drainage were both related to the minimal incisions. Because the surgical field was magnified by the thoracoscope, it was easy for the surgeons to check the cardiac incision and chest cavity more carefully and to ensure that hemostasis was achieved before the closure of the incision. Easy hemostasis, less blood loss and drainage not only reduced the need of blood transfusion and the application of hemostasis materials, but was also cost saving and prevented the patient from the adverse reactions associated with blood infusions (14,15). Before the removal of the aortic cross clamp, adequate de-airing was achieved by pausing of left atrial vent, Trendelenburg position, lung inflation and CO2 insufflation in the operation field. The intraoperative TEE examination also provided us with meaningful information about the valve and de-airing conditions. It was also recommended that the drainage tube was kept in situ for at least two days in order to reduce the incidence of late pleural effusion. No patients developed massive pleural effusions and there were no re-operations for hemostasis in the MT group.
As was mentioned in prior studies, the pain of the TCS patients was considerable reduced compared to that of the conventional sternotomy group (9,16,17). Both VAS and the analgesic administration demonstrated significant pain relief in the MT group. MVR with MTCST through the tri-port at the intercostal space avoided the median sternotomy and maintained the integrity and stability of the sternum. Moreover, very small incisions and the lower post-operative pain levels alleviated the psychological stress of the patients. Postoperative fear might influence the deep breath and not be conducive to the recovery. The mechanical ventilation time, ICU duration and hospital stay were much shorter in the MT group due to the minimal incision, as well as decreased fear, trauma, and pain. The total medical costs of patients undergoing cardiac surgery in China mainly include the operating room costs and the treatment costs during ICU stay, meaning longer ICU duration led to higher costs. The average hospitalization cost in the MT group was lowered by over 2,143 USD compared with the MS group, which was attributed to the reduced blood loss, reduced transfusion, shorter ventilation time and shorter ICU duration.
During follow-up, no cardiac death was found in either MT or the MS group. The LVEF of the patients in the two groups were elevated as compared to their preoperative levels (detailed data is not shown), which further confirms the safety and efficacy of MTCST for MVR. The cosmetic results were appreciated by both female and male patients in the MT group due to the lack of sternal scar. As a result of the decreased surgical trauma, patients undergoing MVR with MTCST returned to normal preoperative jobs or activities earlier than patients with conventional median sternotomy. In summary, the main benefits of MTCST for patients undergoing MVR are less pain, smaller incisions, shorter ICU, and hospital stay, less blood loss and transfusion, and the reduced cost of medical therapy in the hospital. Thus, MTCST was a safe, feasible technique for MVR and a suitable alternative to the MS approach.
Study limitations
There are several limitations in the present study. First, because of the limited indications, it seemed there was a trend for lower weights and female patients in the MT group. Thus, bigger cohort should be studied to avoid the possible bias. Secondly, due to the high morbidity of rheumatic heart disease in China and the complicated procedures of mitral valvoplasty, only MVR was included in present study, with mitral valvoplasty excluded even though this procedure had been carried out with MTCST on quite a few cases. Because MTCST was explored and extended by Dr. Ma, the operations in MT group were performed only by Dr. Ma whereas MS surgeries were performed by other surgeons in the team, which might avoid the differences caused by the skill variation of other surgeons. Furthermore, we merely described the early outcomes with a comparison group of patients undergoing median sternotomy. Long term outcomes should be analyzed and a comparison with right anterolateral thoracotomy or Da Vinci robotic system assistance for MVR should be carried out, even though the latter study is in process currently.
Conclusions
In our more than six years of experience between the years of 2013 to 2019, MTCST without robotic assistance was proved to be a feasible and efficient approach, offering encouraging results for MVR. This novel technique for MVR was associated with a reduced operative blood loss, less blood transfusion, shorter hospital stays and decreased hospitalization costs in comparison with conventional surgery. MTCST is safe and feasible, and it may be a more suitable and desirable minimally invasive alternative to sternotomy in MVR.
Acknowledgments
We acknowledged all the cooperating surgeons for their enormous contributions in data collection. This study was supported by The National Natural Science foundation of China (No. 81600293; No. 81500367) and The Key Research and Development Projects of Shandong Province (No. 2018GSF121007; No. 2015GGE27015).
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mihos CG, Santana O, Pineda AM, et al. Right anterior minithoracotomy versus median sternotomy surgery for native mitral valve infective endocarditis. J Heart Valve Dis 2014;23:343-9. [PubMed]
- Tang P, Onaitis M, Desai B, et al. Minithoracotomy versus sternotomy for mitral surgery in patients with chronic renal impairment: a propensity-matched study. Innovations 2013;8:325-31. [Crossref] [PubMed]
- Geuzebroek GS, Bentala M, Molhoek SG, et al. Totally thoracoscopic left atrial Maze: standardized, effective and safe. Interact CardioVasc Thorac Surg 2016;22:259-64. [Crossref] [PubMed]
- Suri RM, Dearani JA, Mihaljevic T, et al. Mitral valve repair using robotic technology: Safe, effective, and durable. J Thorac Cardiovasc Surg 2016;151:1450-4. [Crossref] [PubMed]
- Suri RM, Taggarse A, Burkhart HM, et al. Robotic mitral valve repair for simple and complex degenerative disease: mid-term clinical and echocardiographic quality outcomes. Circulation 2015;132:1961-8. [Crossref] [PubMed]
- Lewis CT, Stephens RL, Tyndal CM, et al. Concomitant robotic mitral and tricuspid valve repair: technique and early experience. Ann Thorac Surg. 2014;97:782-7. [Crossref] [PubMed]
- Ma ZS, Yang CY, Dong MF, et al. Totally thoracoscopic closure of ventricular septal defect without arobotically assisted surgical system: A summary of 119 cases. J Thorac Cardiovasc Surg 2014;147:863-7. [Crossref] [PubMed]
- Zhe Z, Kun H, Xuezeng X, et al. Totally thoracoscopic versus open surgery for closure of atrial septal defect: propensity-score matched comparison. Heart Surg Forum 2014;17:E227-31. [Crossref] [PubMed]
- Ma ZS, Dong MF, Yin QY, et al. Totally thoracoscopic repair of atrial septal defect without robotic assistance: A single-center experience. J Thorac Cardiovasc Surg 2011;141:1380-3. [Crossref] [PubMed]
- Greco E, Zaballos JM, Alvarez L, et al. Video-assisted mitral surgery through a micro-access: a safe and reliable reality in the current era. J Heart Valve Dis 2008;17:48-53. [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. From classical sternotomy to truly endoscopic mitral valve surgery: a step by step procedure. Heart Lung Circ 2003;12:172-7. [Crossref] [PubMed]
- Moscarelli M, Casula R, Speziale G, et al. Can we use minimally invasive mitral valve surgery as a safe alternative to sternotomy in high-risk patients?. Interact Cardiovasc Thorac Surg 2016;22:92-6. [Crossref] [PubMed]
- Bolotin G, Kypson AP, Reade CC, et al. Should a video-assisted mini-thoracotomy be the approach of choice for reoperative mitral valve surgery?. J Heart Valve Dis 2004;13:155-8. [PubMed]
- Tuinman PR, Vlaar AP, Cornet AD, et al. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: A case control study. Crit Care 2011;15:R59. [Crossref] [PubMed]
- Yu PJ, Cassiere HA, Dellis SL, et al. Dose-dependent effects of intraoperative low volume red blood cell transfusions on postoperative outcomes in cardiac surgery patients. J Cardiothorac Vasc Anesth 2014;28:1545-9. [Crossref] [PubMed]
- Liu G, Qiao Y, Zou C, et al. Totally thoracoscopic surgical treatment for atrial septal defect: mid-term follow-up results in 45 consecutive patients. Heart Lung Circ 2013;22:88-91. [Crossref] [PubMed]
- Ma ZS, Dong MF, Yin QY, et al. Totally thoracoscopic repair of ventricular septal defect: A short-term clinical observation on safety and feasibility. J Thorac Cardiovasc Surg 2011;142:850-4. [Crossref] [PubMed]






