Insertion of the total artificial heart in the Fontan circulation
Introduction
Surgical palliation for a child born with a functionally univentricular heart consists of a series of procedures culminating in an arrangement whereby all systemic venous blood flows through the lungs passively, i.e., without the addition of hydraulic power normally provided by a subpulmonary ventricle. This arrangement is commonly termed the Fontan circulation since its first successful application was described by Fontan in 1968 (1), and may also be called a total cavopulmonary connection. For most patients, the Fontan circulation is durably effective, providing decades of satisfactory performance. However, in some patients, circulatory insufficiency may develop, a condition termed the “failing Fontan”. The manifestations of Fontan failure are generally of two types, somewhat analogous to the manifestations of left heart failure and right heart failure (2). In the former, symptoms are related to inadequacy of the single ventricle, with or without associated atrioventricular or semilunar valve dysfunction, and are those of inadequate systemic cardiac output. In the latter, symptoms are related to inability to achieve adequate pulmonary blood flow at acceptable central venous pressures, and typically include peripheral edema, ascites, pleural effusions, protein-losing-enteropathy (PLE), and more rarely, plastic bronchitis (PB). For completeness, it should be mentioned that the latter two conditions, PLE and PB, may represent a third type of Fontan failure, that related to disorders of lymphatic drainage and connection, exacerbated by elevated central venous pressure (3). It is important to emphasize that the various forms of Fontan failure rarely occur in isolation, and many patients will have some manifestations of left and right heart-type symptoms, occasionally complicated by PLE or PB or both.
For patients whose “Fontan failure” is incompletely responsive to medical therapy, at present the only definitive treatment option is heart transplantation. As with other types of patients with heart failure, the time between listing for heart transplantation and actually receiving an organ is one of great risk for both death and significant clinical deterioration, the latter of which may reduce the likelihood of successful transplant outcome. In patients with biventricular circulation, this risk of death or clinical deterioration may be mitigated with the use of mechanical circulatory support as a bridge to transplant, most commonly in the form of left ventricular assist devices (LVADs), now used in between 40% and 50% of adult patients undergoing transplantation (4). There has been some limited success with the use of such devices in patients with the Fontan circulation (5). Intuitively, this is most likely to be practical in patients who have the “purest” form of left heart failure, and least likely to be helpful in those with preserved systemic ventricular function, i.e., right-sided failure symptoms. That said, there are case reports of success in both forms of Fontan failure (5). However, for patients with true right and left heart symptoms, mechanical support of both the pulmonary and systemic circulations is desirable. For such patients, the only current FDA-approved device for “biventricular support” is the SynCardia Total Artificial Heart (TAH) (6,7), which is available in two pump sizes (50 and 70 cc) (SynCardia, Tucson, Arizona, USA). In this paper, we will review the surgical steps necessary for implantation of the TAH in patients with the Fontan circulation.
Operative techniques
Preparation
Preparation for TAH implantation in a Fontan patient begins with a complete evaluation of all pathways and connections in the actual Fontan construction, as well as assessing the patency of the central veins, pulmonary arterial tree, pulmonary veins, and the systemic arteries. In addition, the specific morphology of the systemic ventricle(s) and atrioventricular valve anatomy must be understood completely. Computed tomography permits virtual fit testing to determine which pump, if any, will fit the patient (8). It also demonstrates areas of calcification (such as in patch material in the aorta and aortic arch) and any structures at particularly high risk during sternal re-entry. Magnetic resonance imaging provides a reasonable estimate of absolute cardiac output as well as absolute systemic to pulmonary arterial collateral flow. These data have important implications for the conduct of cardiopulmonary bypass during implant (predicting optimal flow rate) and for determining if a maximally operating TAH can provide close to the total cardiac output required for appropriate end-organ perfusion. Finally, it is imperative to determine sites available for peripheral cannulation. In aggregate, this survey will enable the surgeon to plan the conduct of cardiopulmonary bypass to permit implantation of the TAH as well as to address any “residual” hemodynamic abnormalities such as aortic arch obstruction or branch pulmonary artery stenosis.
Exposition
Type of Fontan connection
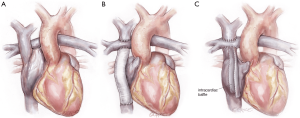
The Fontan circulation has been most commonly achieved as an “atriopulmonary Fontan”, a “lateral tunnel Fontan”, or an “extracardiac Fontan” (Figure 1) (9). The operating surgeon must understand surgical details of how the Fontan was achieved, because to perform the TAH implant the Fontan must be “taken down” in order to achieve a site for the connection to the right-sided pump, for both inflow and outflow. An important detail for lateral tunnel patients is to determine whether the second stage operation was performed as a bidirectional Glenn connection or a hemi-Fontan procedure (10).
Type of ventricular morphology
Many patients with Fontan circulation will indeed have a univentricular heart, with a single atrioventricular valve. In such patients, attachment of the left-sided inflow “quick connect-atrial cuff” is similar to attachment of the left-side in a patient with a biventricular circulation (11). Other patients with Fontan circulation may actually have two ventricles and even two atrioventricular valves. In such patients, the “extra” ventricle or valve will need to be accounted for in attaching the atrial quick connect. This may involve closure of an atrioventricular valve, resection of the ventricular septum, or other procedure tailored to the unique aspects of patient anatomy.
Operation
- Redo sternotomy and preliminary dissection—all Fontan patients will have had at least one prior sternotomy, and most will have had at least two. Safe sternal re-entry and preliminary dissection are mandatory. In certain cases, it may be prudent to expose peripheral vessels before attempting sternal re-entry, depending on the perceived risk of catastrophic re-entry injury. The first portion of the intrathoracic dissection involves the exposure of the superior and inferior vena cavae, with sufficient length on both to permit cannulation and Fontan takedown. The central pulmonary artery should also be exposed sufficiently to allow reconstruction after takedown and attachment to the right-sided pump. Likewise, the ascending aorta must be mobilized enough to permit cannulation and cross-clamping with sufficient length for anastomosis to the left-sided pump. Ideally, all of this preparation is accomplished off cardiopulmonary bypass. However, patient instability or other factors such as dense adhesions may require cannulation at alternative sites to either complete the dissection or allow for adequate exposure of the inferior vena cava for bicaval venous cannulation.
- With preliminary dissection completed, standard aortic and bicaval cannulation is accomplished (alternatively, the innominate vein may be cannulated rather than superior vena cava), with placement of a left-sided vent to capture the pulmonary venous return, which is frequently torrential in Fontan patients as a consequence of systemic to pulmonary collateral artery flow. Early venting is particularly important for the patient with severely diminished ventricular function and a regurgitant neo-aortic valve as fibrillation could lead to acute pulmonary edema. A moderate degree of hypothermia is typically used for both organ preservation and to permit very brief periods of low flow or even circulatory arrest. The aorta is cross-clamped to eliminate flow to the myocardium and coronary sinus return.
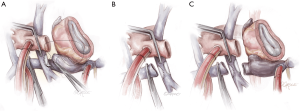
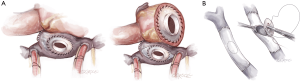
- Fontan takedown is then begun, tailored to the type of Fontan construction. In all cases, the systemic venous circulation will need to be detached from the pulmonary artery, with resultant defect(s) in the pulmonary artery addressed (Figure 2). In the case of an atriopulmonary Fontan, there will be a single pulmonary arterial defect. This may be patched, or left as the site for connection to the outflow from the right-sided pump. For extracardiac Fontan patients, two defects will result from takedown, and the superior one must be patched. The inferior defect is either patched for used or connection as with the atriopulmonary setup. Management of the lateral tunnel Fontan connection must be individualized, depending on the technique used for the superior cavopulmonary connection (bidirectional Glenn or hemi-Fontan). In the former case, the takedown and reconstruction will be similar to an extracardiac circumstance. In the setting of a hemi-Fontan/lateral tunnel, the pulmonary arterial reconstruction will likely be more extensive.
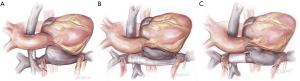
- Restoring systemic venous continuity is the next step for patients with lateral tunnel or extracardiac Fontan (Figure 3). In the latter case it is perhaps simplest to excise the original tube graft and to place a new, large diameter polytetrafluoroethylene PTFE graft to connect the superior and inferior vena cavae (6). In the lateral tunnel patient, the SVC can be connected to the original right atrium or superior cavoatrial junction with an interposition PTFE graft. For patients with an atriopulmonary Fontan, wherein both cavae were left attached to the right atrium, this step is not necessary. In all types of Fontan, consideration should be given to eliminating any residual shunts (fenestration or atrial septal defect) at this point, to prevent right to left shunting or paradoxical embolism after TAH implantation.
- The systemic ventricle(s) and atrioventricular valve tissue are then excised in order to create a substrate for attachment of the left-sided atrial quick connect (Figure 4). For true single ventricle patients with a single atrioventricular valve, this step is straightforward and analogous to the procedure in a patient with biventricular anatomy (11). For patients with two ventricles, two atrioventricular valves, or both, an alternative solution is required. In some cases, excision of the second valve to create a single AV orifice may suffice. In other patients complete excision of the ventricular tissue, with attachment of the atrial quick connect to atrial tissue may be the best solution (7). If the atrial chamber is large enough or can be made large enough to be partitioned, attachment of both the left and right-sided quick connects can be made at the atrial level (7). For the 50 cc TAH, atrial quick connect cuffs should not be trimmed excessively, at least for the right-sided pump. The 50 cc TAH is a down-sized pump with the angle between the inflow and outflow the same as the 70 cc pump. Cutting the cuff too short could lead to an inadequate space for the right-sided outflow graft to pass over the left-sided outflow graft without posterior effacement.
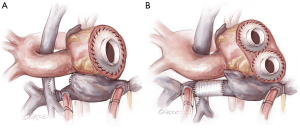
- The next step is to attach the right-sided inflow quick connect (Figure 5). If an SVC to IVC graft has been inserted, a defect is created in the graft wall, to which the quick connect is anastomosed (6). If there was a lateral tunnel Fontan, a right atriotomy is fashioned, to which the quick connect atrial cuff is anastomosed. If the lateral tunnel channel is relatively small, the edges of the atriotomy may be lengthened with patch material.
- Following this, the outflow grafts are attached to the aortic remnant and to the pulmonary artery. The latter step must be done with careful planning to ensure that the site of attachment will permit a non-distorted configuration for the right-sided pump. In certain patients with a very short ascending aorta (common in Fontan patients), there will be limited tissue below the aortic clamp. In this case, or if the tissue integrity is poor, the aortic anastomosis may require a period of circulatory arrest.
- With both inflow and outflow connectors securely anastomosed on each side, left and right pumps are inserted and the drivelines tunneled (11). This portion of the procedure is essentially identical to that for patients with biventricular hearts. After de-airing is completed, weaning from bypass should occur uneventfully.
- The final steps in the procedure are the achievement of hemostasis, which must be meticulous, and the preparation for the transplant operation (12). This latter concept will include the judicious placement of PTFE membranes to facilitate re-entry and mobilization of the components of the TAH. Purposeful placement of material to facilitate exposure of the vena cavae and great vessels will be greatly appreciated by the surgeon who performs the subsequent heart transplantation.
- Special considerations.
- Intraoperative bleeding
While severe bleeding occurs in many types of cardiac surgery, it can be especially troublesome after implanting a TAH in a failing Fontan, in whom the ability of the liver to produce normal levels of clotting factors may impaired. The following are points to consider which may help minimize bleeding-related morbidity. - Most patients will either have been on warfarin and/or some antiplatelet agent. Both aspects of coagulation should be normalized prior to skin incision.
- Severe post-protamine bleeding requires aggressive product, platelet, and red cell support using a rapid infusion system. Coagulation concentrates and prothrombin complex are useful adjuncts.
- Generous use of hemostatic packing, including securing packing material to the sternal margin tightly with suture, with planned delayed sternal closure is a prudent option in many cases. This avoids excessive bleeding the first night, and packing can be removed the next day without recurrence of bleeding.
- Vasoplegia
Vasoplegia in the Fontan patient shares etiologic aspects with that occurring in other heart failure patients (13), with one additional factor. Extensive systemic to pulmonary collateral flow, which is the rule rather than the exception in Fontan patients, can worsen or solely account for what appears to be vasoplegia. While the usual anti-vasoplegia medications can be used, the best option is to increase device flow. If this does not work, accessible collaterals should be embolized by early cardiac catheterization with the understanding that impact can be difficult to predict. It can range from absolutely no impact to immediate improvement in blood pressure and end-organ perfusion.
Comments
There is very limited experience with the use of the TAH in Fontan patients, which is doubtless due to the perceived magnitude of the operation as well as the typically severely debilitated state of the candidate patients. The legitimacy of these concerns is attested to by the typically-observed high morbidity and mortality in patients undergoing transplant for failing Fontan. This is not surprising in that the process is appropriately described as performance of a long, complex operation in a debilitated patient who is immediately exposed to immunosuppression. By contrast, support of the failing Fontan patient with the TAH permits the transplant operation to be simpler. Pre-transplant reconstruction is accomplished during device implant, end-organ recovery can begin before exposure to immunosuppression, nutrition can be optimized, and rehabilitation can be undertaken. Furthermore, if a patient does not survive the TAH implant, it may be speculated that post-transplant survival would have been even less likely. This, in effect, is the ultimate selection process and may prevent a futile attempt at transplant.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240-8. [Crossref] [PubMed]
- Book WM, Gerardin J, Saraf A, et al. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis 2016;11:296-308. [Crossref] [PubMed]
- Biko DM, DeWitt AG, Pinto EM, et al. MRI evaluation of lymphatic abnormalities in the neck and thorax after Fontan surgery: relationship with outcome. Radiology 2019;291:774-80. [Crossref] [PubMed]
- Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056-66. [Crossref] [PubMed]
- Carlo WF, Villa CR, Lal AK, et al. Ventricular assist device use in single ventricle congenital heart disease. Pediatr Transplant 2017. [Crossref] [PubMed]
- Rossano JW, Goldberg DJ, Fuller S, et al. Successful use of the total artificial heart in the failing Fontan circulation. Ann Thorac Surg 2014;97:1438-40. [Crossref] [PubMed]
- Woods RK, Niebler R, Kindel S, et al. A new method for implanting a total artifical heart in the patient with a Fontan circulation. J Thorac Cardiovasc Surg 2019;157:353-5. [Crossref] [PubMed]
- Ferng AS, Oliva I, Jokerst C, et al. Translation of first north American 50 and 70 cc total artificial heart virtual and clinical implantations: utility of 3D computed tomography to test fit devices. Artif Organs 2017;41:727-34. [Crossref] [PubMed]
- de Leval MR. The Fontan circulation: a challenge to William Harvey? Nat Clin Pract Cardiovasc Med 2005;2:202-8. [Crossref] [PubMed]
- Herrmann JL, Brown JW. The superior cavopulmonary connection: history and current perspectives. World J Pediatr Congenit Heart Surg 2019;10:216-22. [Crossref] [PubMed]
- Arabia FA, Copeland JG, Pavie A, et al. Implantation technique for the CardioWest total artificial heart. Ann Thorac Surg 1999;68:698-704. [Crossref] [PubMed]
- Ihnken KA, Ramzy D, Esmailian F, et al. Surgical technique to facilitate explantation of mechanical circulatory support devices: LVADs, BiVADs, and TAHs before heart transplantation. ASAIO J 2016;62:211-3. [PubMed]
- van Vessem ME, Palmen M, Couperus LE, et al. Incidence and predictors of vasoplegia after heart failure surgery. Eur J Cardiothorac Surg 2017;51:532-8. [PubMed]






