Heart transplantation after SynCardia® total artificial heart implantation
Introduction
Demographic factors and improving survival of patients with cardiovascular disease has seen an increase in the prevalence of heart failure (HF). In Germany, for example, the number of hospital referrals due to HF in individuals older than 65 years has increased. In contrast, death rates due to HF have decreased from 63.7 per 100,000, to 42.8 per 100,000 inhabitants per year in 2006 and 2016, respectively. This is primarily explained by improved therapeutic options (1,2).
Over the previous decade, the implantation of durable mechanical circulatory support (MCS) devices has evolved towards a well-established treatment option for patients with therapy refractory terminal HF. The majority of such patients can effectively be treated with implantation of a left ventricular assist device (LVAD), yielding improved survival, quality of life, and exercise capacity, with relatively low complication rates (3,4). However, there is a certain portion of patients, probably further down the road of HF, with additional, significant dysfunction of the right heart (3,4).
Durable biventricular MCS is a challenging task. Modern LVADs are not approved for right ventricular support, although expertise is slowly growing for the use of these continuous flow pumps in right ventricular assistance (5). Remaining approved options are the implantation of two paracorporeal Berlin Heart Excor® Systems for right and left ventricular assistance, or the SynCardia TAH®, which is the only approved total artificial heart (TAH) system. In smaller numbers of terminal HF patients with ventricular thrombosis, unfavourably small ventricular cavities, for example, in restrictive or concentric-hypertrophic cardiomyopathies, cardiac tumors or certain congenital conditions, removal of the ventricular portions and implantation of a TAH is the only durable MCS option (6).
Although the SynCardia TAH® is completely implantable, it requires percutaneously guided air conduction tubes connected to an extracorporeal driver system. As it is a pneumatic system, both the “Companion”-driver for in-hospital and the bulky “Freedom”-driver for ambulatory use produce noise which may disturb the patient and impact quality of life. Different to LVAD systems, malfunction of the device may lead to immediate death in SynCardia TAH® patients. Therefore, the SynCardia TAH® is generally implanted with a bridge-to-transplant indication.
The aim of this report is to analyse the outcome of SynCardia TAH® patients after cardiac transplantation in a high volume MCS and heart transplant centre. Further, technological aspects of the demanding transplant procedure and the current significance of SynCardia TAH® therapy in terminal HF and transplant candidates are discussed.
Methods
Study design and patients
In a retrospective, single-centre analysis, all patients receiving a left-ventricular (LVAD) or biventricular assist device (BVAD), a SynCardia TAH® or solely a heart transplantation without prior MCS therapy between January 2001 and December 2019 were analyzed, focusing on comparison of outcomes after heart transplantation in TAH patients. Written and informed consent for scientific use of clinical data was routinely obtained by all patients and their relatives (in case of unconscious patients). The investigation conforms to the principles outlined in the Declaration of Helsinki. Because of the retrospective study design, the need for an Ethics committee approval was waived. Baseline and postoperative patient characteristics as well as clinical follow up data of the patients were available through a prospectively maintained database of our MCS program. The primary endpoint was survival after heart transplantation during follow up.
Statistics
Kaplan Meier survival estimates were calculated for all four patient groups. Cox regression analysis was used to compare clinical outcomes between the study groups. Data are given in absolute numbers, percentages, or mean values ± standard deviation (range), as indicated. P values <0.05 were considered statistically significant. We applied the statistical software package IBM SPSS, version 24 (IBM Corp, Armonk, NY, USA), R (version 2.15.3), and the SPE file in SPSS to perform the analyses.
Results
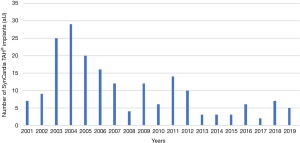
Retrospective data analyses revealed that n=193 patients were implanted with a SynCardia TAH® system at our center between the years 2001 and 2019. The primary indications for implantation are listed in Table 1. Ischemic cardiomyopathy was the leading diagnosis in SynCardia TAH® candidates. The number of implants per year declined over time (Figure 1).
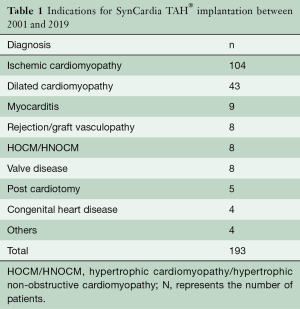
Full table
A total of n=69 SynCardia TAH® patients were transplanted at our centre (37.3% of all SynCardia TAH® implants) during the study period. The majority of SynCardia TAH® patients transplanted were categorized as Eurotransplant high urgency (HU)-status (81.2%), while fewer patients were transplanted in the regular transplantable (T) status (15.9%; Table 2). The mean time on SynCardia TAH® support was 303±198 days (1–733 days).
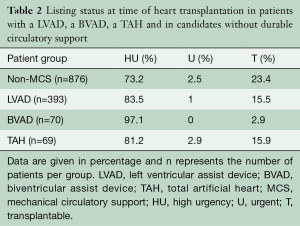
Full table
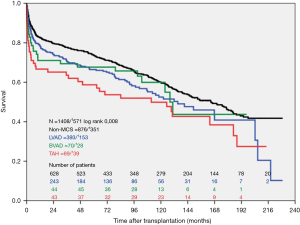
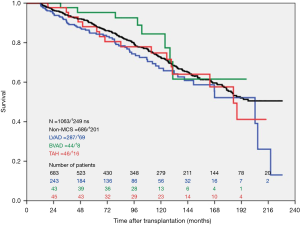
During the study period, n=393 LVAD patients, n=70 BVAD patients and n=876 non-MCS patients were transplanted at our high-volume heart center. The actuarial survival per group after heart transplantation is depicted in Figure 2. Survival after heart transplantation was significantly lower in SynCardia TAH® patients (P=0.008) when compared to the other groups. Survival after heart transplantation was not affected by the primary diagnosis at the time of SynCardia TAH® implantation (P>0.05). Multi-Organ failure was the predominant cause of death in approximately one third of all transplanted SynCardia TAH® patients (data not shown). Survival after heart transplantation was not significantly different between SynCardia TAH® (n=46), BVAD (n=44), LVAD (n=287) and non-MCS patients (n=686) who survived the 1st year after transplantation (Figure 3).
Discussion
This study analyses survival after heart transplantation in SynCardia TAH® patients and shows impaired survival rates in these patients compared to patients transplanted after LVAD- and BVAD implantation and non-MCS transplant patients.
Obviously, SynCardia TAH® patients represent an especially risky patient cohort for cardiac transplantation. It seems logical, that the initial diagnosis at device implantation does not affect outcome after transplantation. In fact, as the heart is virtually completely removed during device implantation, only the systemic impact of the underlying disease could affect outcomes after heart transplantation in SynCardia TAH® patients.
The Intermacs registry data on the TAH show better transplant rates and transplant outcomes in TAH patients compared to our single center analyses (6), which may be explained by our less liberal use of the TAH in durable MCS therapy. In addition, there is a dramatic lack of organ donations in Germany and different allocation policies. As a consequence of the latter, we handle relatively poorer donor organ quality, for example in view of donor age, which is an acknowledged risk factor for outcome after transplantation (7). In a prospective, investigator-initiated trial, Copeland and coworkers reported superior 1- and 5-year survival rates in transplanted TAH patients as herein of 86% and 64%, respectively (8). However, when interpreting these results, one has to acknowledge that, apart from potential donor organ quality matters, the study design was different to our “real world” retrospective analysis with distinct inclusion and exclusion criteria.
Obviously, there is a high mortality rate in transplanted SynCardia TAH® patients early post-transplant. But it is interesting to note that after surviving the first 12 month posttransplant, long-term survival of these transplanted SynCardia TAH® patients parallels the outcome in transplanted LVAD-, BVAD- or non-MCS patients. Thus, heart transplantation may very well be a reasonable option after SynCardia TAH® implantation, but obviously requires vigorous risk stratification. It remains an open question, which SynCardia TAH® patients carry a high risk for early death and why the perioperative period is critical in these patients? In-fact, listing for heart transplantation mandates a “transplantable” condition generally in all candidates. In Germany, the high-urgency status is warranted in MCS patients, only if life-threatening complications of the MCS device occur, e.g. infection or thrombosis of the device. One could argue that the majority of SynCardia TAH® patients are transplanted in HU-status, thus being in worse condition, but this holds true also for all other patient groups due to the dramatic organ shortage. In other words, there is an as-yet undefined significant risk in TAH patients during heart transplantation directly linked to this TAH technology.
SynCardia TAH® cannot be completely evaluated for transplantation. For example, the device does not allow for direct measurement of pulmonary artery pressures and pulmonary resistance, since trans-ventricular passage with a pulmonary artery catheter is not possible. Maybe modern tools for indirect measurements, such as the CardioMEMS®, may potentially help to uncover high-risk conditions such as significant pulmonary vascular disease prior to transplant (9).
Another critical aspect to the success of transplant after the SynCardia TAH® is the surgical procedure for device implantation. In our experience, formation of adhesive tissues after SynCardia TAH® implantation is excessive and is distinctively worse than after LVAD implantation. Although this notion cannot be supported by conclusive data analyses, we noticed tremendous thickening of the pericardium, most probably as an inflammatory reaction to the particular device. This is not regularly met in transplant candidates with other MCS devices. As a consequence, we perform SynCardia TAH® implantation with intensive coverage of all device parts with a synthetic Goretex® membrane (10,11). It is recommended that all vascular structures shall be covered in order to avoid adhesions and to facilitate device explantation and transplantation (11). Further, the shape of the SynCardia TAH® device is significantly different from a native heart and therefore the intra-mediastinal adhesive changes complicate imbedding of the heart graft. Extensive pericardial and pleural resection may be necessary.
In addition, the time-consuming surgical preparations may lead to prolonged cold and warm ischemic times. As such, thorough logistical planning and performance is absolutely required when transplanting a Syncardia TAH® patient. It remains to be determined whether artificial machine perfusion during organ procurement and transportation may be helpful to reduce ischemic times and allow for extended recipient preparations, thus improving outcomes (12).
In summary, implantation of the SynCardia TAH® system should only be undertaken where clearly indicated and other durable MCS devices are not suitable, as long-term survival in our series was poorer in TAH patients when compared to other MCS and non-MCS patients. The less favorable quality of life and adverse event profile of the system must be considered when scheduling patients for the bridge-to-transplant. The latter and the better survival on other durable MCS devices make the SynCardia TAH® inadvisable for destination therapy. Heart transplantation in SynCardia TAH® patients must be carefully planned, with particular attention paid to the TAH implant procedure, mid-term surveillance and transplant surgery. TAH implantation should preferably be performed in centers with an experienced high-volume MCS and heart transplant program (6). TAH implantation and subsequent heart transplantation requires distinct risk stratification to improve outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The need for an Ethics Committee approval was waived and written informed consent was obtained from all patients and their relatives.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Kommentar zu den Leitlinien der Europäischen Gesellschaft für Kardiologie (ESC) zur Diagnostik und Behandlung der akuten und chronischen Herzinsuffizienz. Available online: https://leitlinien.dgk.org/files/2016_K_Herzinsuffizienz.pdf
- de By TMMH, Mohacsi P, Gahl B, et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): second report. Eur J Cardiothorac Surg 2018;53:309-16. [Crossref] [PubMed]
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080-6. [Crossref] [PubMed]
- Schramm R, Morshuis M, Schoenbrodt M, et al. Current perspectives on mechanical circulatory support. Eur J Cardiothorac Surg 2019;55:i31-7. [Crossref] [PubMed]
- Arabía FA, Cantor RS, Koehl DA, et al. Interagency registry for mechanically assisted circulatory support report on the total artificial heart. J Heart Lung Transplant 2018;37:1304-12. [Crossref] [PubMed]
- Joyce DL, Li Z, Edwards LB, et al. Predicting 1-year cardiac transplantation survival using a donor-recipient risk-assessment tool. J Thorac Cardiovasc Surg 2018;155:1580-90. [Crossref] [PubMed]
- Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351:859-67. [Crossref] [PubMed]
- Veenis JF, Birim O, Brugts JJ. Pulmonary artery pressure telemonitoring by CardioMEMS in a patient pre- and post-left ventricular assist device implantation. Eur J Cardiothorac Surg 2019;56:809-10. [Crossref] [PubMed]
- Copeland JG, Arabia FA, Tsau PH, et al. Total artificial hearts: bridge to transplantation. Cardiol Clin 2003;21:101-13. [Crossref] [PubMed]
- Ihnken KA, Ramzy D, Esmailian F, et al. Surgical Technique to Facilitate Explantation of Mechanical Circulatory Support Devices: LVADs, BiVADs, and TAHs Before Heart Transplantation. ASAIO J 2016;62:211-3. [PubMed]
- Monteagudo Vela M, García Sáez D, Simon AR. Current approaches in retrieval and heart preservation. Ann Cardiothorac Surg 2018;7:67-74. [Crossref] [PubMed]






