Sternotomy versus thoracotomy lung transplantation: key tips and contemporary results
Introduction
The procedure of lung transplantation has evolved significantly over the last 40 years. The most significant technical change has been from a tracheal anastomosis to a hilar anastomosis in order to avoid bronchial ischemia (1). Details of the operative steps performed vary by surgeon and institution. No matter the approach, an emphasis on early extubation, excellent pulmonary toilet and aggressive ambulation of the patient postoperatively is critical.
Despite the frequent use of both incisions, few studies have compared outcomes after a clamshell thoracotomy (bilateral anterior submammary thoracotomies connected by a transverse sternotomy) versus a median sternotomy for performing bilateral lung transplantation. Better pulmonary function and less pain have been described with sequential bilateral anterolateral thoracotomies compared to the clamshell incision (2). Notably, exposure of the mediastinum may be compromised without division of the sternum in this technique. Median sternotomy for lung transplantation has been well described (3-6) and may allow for a shorter operative time, improved chest wall mechanics and fewer wound complications (7). Additionally, the sternotomy approach frequently requires the use of cardiopulmonary bypass for hemodynamic support, to permit exposure for the hilar anastomosis. Given concerns regarding resuscitative needs post bypass and resulting primary graft dysfunction, a median sternotomy has been avoided in some institutions.
In our experience, routine use of cardiopulmonary bypass allows improved intraoperative hemodynamic stability, decreased warm ischemic time and better controlled cold reperfusion; consequently, the median sternotomy approach to lung transplantation has superior short-term results and equivalent midterm results compared to other incisions. Therefore, we present our operative technique of a sternotomy approach to lung transplant surgery and its results in order to update the literature on this topic.
Operative techniques
Preparation
There are a few contraindications to the sternotomy approach. These include enlarged hilar lymph nodes from conditions such as sarcoidosis, which complicates the vascular anastomoses. A preoperative CT angiogram should be obtained in these cases to examine the relationship of the calcified lymph nodes to the pulmonary artery (PA). Furthermore, posterior-apical pleural thickening, such as in patients with cystic fibrosis, can be difficult to reach through a median sternotomy. Patients with pulmonary fibrosis and obstructive disease are ideal for the sternotomy approach, as there are few adhesions and the hila are very well visualized through sternotomy.
Exposition
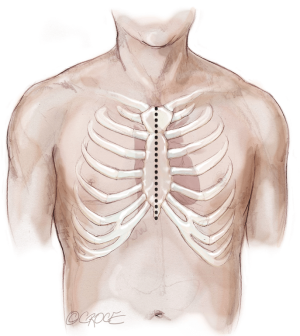
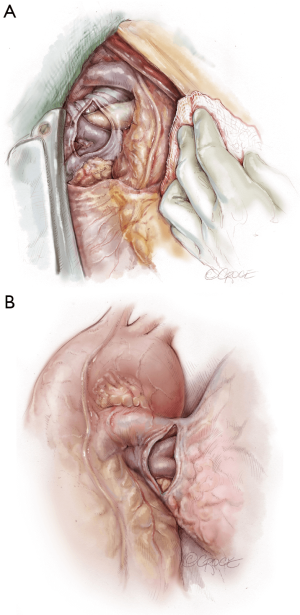
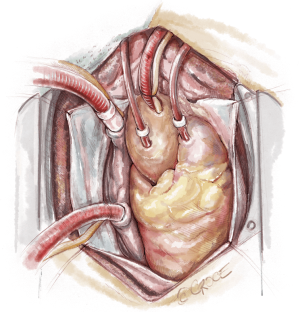
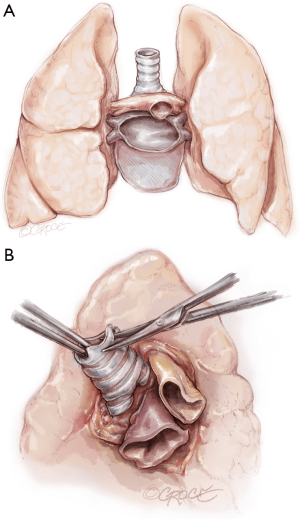
The recipient is placed in a supine position with their arms tucked. The neck is extended using a shoulder roll. A midline sternotomy is made (Figure 1). The pleura is opened bilaterally. The chest is explored for adhesions, which are freed with electrocautery to ensure hemostasis. The anterior aspect of the hila are prepared by dissecting the superior pulmonary vein and main branch pulmonary arteries, taking care to avoid the phrenic nerve (Figure 2A,B), which is in close proximity to the hilum particularly on the right side. Next, a pericardial well is established, heparin is administered and full cardiopulmonary bypass is initiated with ascending aorta and bicaval cannulation. Superior vena cava cannulation is important to avoid poor upper body drainage during retraction to expose the right hilum. An aortic root vent and PA vent are added (Figure 3). The temperature is allowed to drift to 32 degrees.
Operation
The hilar dissection proceeds and both pneumonectomies are performed. We staple the vascular structures with an endo-GIA stapler. Attention is paid to hemostasis and controlling lymphatic channels with clips. On the right, the hilar lymph node packet is maintained intact by hugging the bronchus intermedius with the electrocautery. Devascularizing the native bronchus is avoided. The opened bronchus is suctioned clean and silk stay sutures are placed at the junction of the cartilaginous and membranous portions to aid retraction during the anastomosis (Figure 4). The pericardium encircling the PA and left atrium is opened wide in order to lengthen and free these structures.
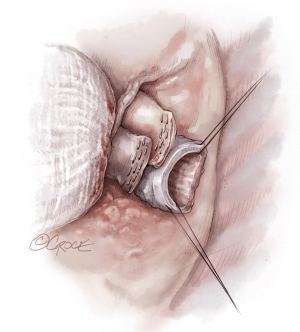
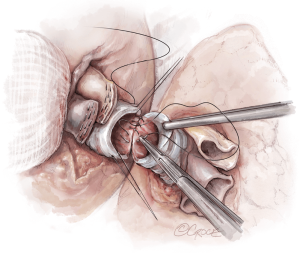
Once hemostasis of the chest wall has been confirmed and the hila are prepared for implantation, the donor lungs are prepared. The left atrial cuff and pulmonary arteries are divided and trimmed. The donor bronchus is divided two rings above the secondary carina in order to avoid ischemia (Figure 5A,B).
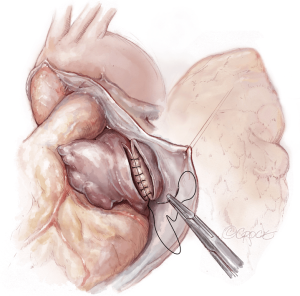
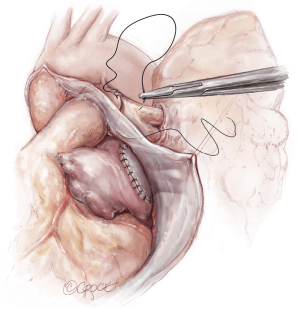
The lungs are implanted sequentially, starting with the bronchus using a long continuous running 4-0 Prolene on a SH-1 needle. The anastomosis is done end to end, starting with the posterior membranous portion (Figure 6). The same type of suture is used for the pulmonary vein anastomosis, which is done intrapericardially on the left (Figure 7). Continuous running 5-0 Prolene on a RB-1 needle is used for the PA (Figure 8). On the right, a vascular clamp is applied during the posterior wall of the PA anastomosis, to avoid retraction behind the SVC. The PA vent rate can be modulated to keep the field dry and also allow the first lung to be partially perfused once implanted. The second lung is then implanted in a similar fashion. We add a left atrial vent across the second pulmonary vein anastomosis to aid in de-airing.
Completion
Bronchoscopy is used to examine and clear the airways before ventilating with low tidal volumes and room air. After a period of cold reperfusion and deairing, transesophageal echocardiogram is done to confirm pulmonary vein flow and the patient is warmed and weaned from bypass. Heparin is reversed with protamine. Chest drains are placed in bilateral hemithoraces and the mediastinum. Closure is done with an overlapping figure of eight sternal wires.
Comments
Clinical results
We examined short term outcomes of 71 bilateral lung transplant cases done at Brigham and Women’s Hospital from October 2013 to January 2017, of which 31 were done by clamshell and 40 by sternotomy. Average case duration (358 vs. 473 minutes) and ischemic time (248 vs. 347 minutes) were significantly shorter in the sternotomy group (P<0.001). Transfusion requirements were significantly higher for clamshell group with 3.52 vs. 0.85 units PRBC (P<0.001) and 1.81 vs. 0.65 bags of platelets (P=0.05). Time to extubation was significantly faster for sternotomy patients with 70% (28 of 40) extubated by 48 hours postop compared to 39% (n=11/28) (P=0.003). Sternotomy maintained statistical significance for association with less transfusions and early extubation when controlling for patient demographics, comorbidities and LAS score. Our study is the largest series comparing clamshell versus sternotomy incisions for bilateral lung transplant and demonstrates improved short-term outcomes after sternotomy incision.
Advantages
Less soft tissue dissection, better pain control, and earlier mobilization are benefits of sternotomy versus clamshell incision for bilateral lung transplantation. In our anecdotal experience, patients also use less narcotics after sternal incision compared to thoracotomy. Furthermore, recognizing an increasingly elderly end stage lung disease population, median sternotomy provides exposure to perform concomitant cardiac procedures as needed at the time of transplant.
With increasing use of marginal donors, sternotomy with cardiopulmonary bypass support also offers the flexibility of central cannulation for postoperative ECMO if needed. We have found that the sternotomy approach maximizes the benefits of excellent exposure of the mediastinum and hilum and full hemodynamic support while minimizing pain and bleeding.
Caveats
More research is needed to measure pain, quality of life and other patient-reported outcomes after a lung transplant performed by various surgical incisions. Future work comparing prospective measurement of primary graft dysfunction and long-term outcomes will be crucial to informing the use of a particular surgical incision.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pasque MK, Cooper JD, Kaiser LR, et al. Improved technique for bilateral lung transplantation: rationale and initial clinical experience. Ann Thorac Surg 1990;49:785-91. [Crossref] [PubMed]
- Meyers BF, Sundaresan RS, Guthrie T, et al. Bilateral sequential lung transplantation without sternal division eliminates posttransplantation sternal complications. J Thorac Cardiovasc Surg 1999;117:358-64. [Crossref] [PubMed]
- Dark JH. Median Sternotomy for Lung Transplantation. Operative Techniques in Thoracic and Cardiovascular Surgery 2015;20:87-103. [Crossref]
- Kohno M, Steinbruchel DA. Median sternotomy for double lung transplantation with cardiopulmonary bypass in seven consecutive patients. Surg Today 2012;42:406-9. [Crossref] [PubMed]
- Teman NR, Xiao JT, Tribble CG, et al. Median Sternotomy for Lung Transplantation: Techniques and Advantages. Heart Surg Forum 2017;20:E089-E091. [Crossref] [PubMed]
- Bates M, Factor M, Parrino PE, et al. Lung Transplantation and the Routine Use of Cardiopulmonary Bypass and Median Sternotomy: Experience at the Ochsner Multi-Organ Transplant Institute. Ochsner J 2017;17:38-41. [PubMed]
- Macchiarini P, Ladurie FL, Cerrina J, et al. Clamshell or sternotomy for double lung or heart-lung transplantation? Eur J Cardiothorac Surg 1999;15:333-9. [Crossref] [PubMed]