Inter-hospital transfer of extracorporeal membrane oxygenation-assisted patients: the hub and spoke network
Introduction
Cardiogenic shock (CS) carries a high risk for morbidity and mortality (1-3). In case CS-patients cannot be stabilized by conservative intensive care means they require acute mechanical circulatory support (MCS). Acute MCS may be established by intra-aortic balloon pulsation (iABP; the Impella® device) or, most effectively, by extracorporeal membrane oxygenation (ECMO). In the veno-arterial setting, ECMO therapy is also designated as extracorporeal life support (ECLS), which is the only system to acutely provide full cardiopulmonary support (4). Strategically, acute MCS may be used (I) as a bridge to recovery, when the causative diagnosis can be effectively treated; (II) as a bridge to implantation of a durable MCS system, e.g., left ventricular assist devices or total artificial hearts; or (III) as a bridge to transplantation, which is a fairly unrealistic option considering the relatively long waiting times due to the global organ shortage. In fact, cardiac transplantation may rather become a long-term goal after bridging therapy with a durable MCS system (5-7).
The availability of acute MCS is limited, particularly in smaller and peripheral hospitals. The use of these devices requires specifically experienced and regularly trained staff, which are only possible to have in larger, experienced centers. As transportation of CS-patients carries a tremendous risk, tertiary care centers have established mobile ECLS teams in order to provide high-end interdisciplinary cardiac care in the remote clinics and to retrieve critically ill patients after acute institution of MCS into tertiary care (4).
Herein, we summarize our single-center experience with a mobile ECLS team program. This report focusses on the procedural algorithms and presents survival data of CS-patients retrieved after on-site institution of an ECLS system and transportation into our center.
Methods
Patients
We retrospectively analyzed survival data of all consecutive CS-patients between January 2012 and October 2018, which were retrieved on acute MCS by our mobile ECLS team from external hospitals into our tertiary care unit. Table 1 summarizes anamnestic parameters routinely prompted during the first contact with the requesting center. Reasons for decline comprised of excessive acidosis (pH <6.9), serum lactate levels >20 mmol/L, poor neurological prognosis and prolonged resuscitation. Overall decision did not underlie clear cut-off parameters.
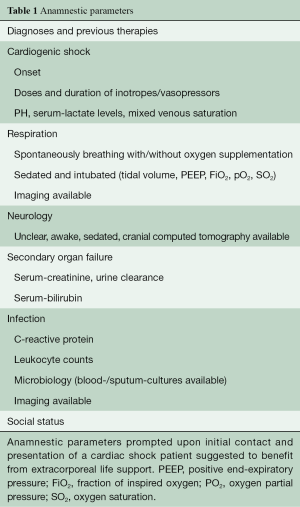
Full table
Interventions
All ECLS systems were instituted on-site percutaneously via the groin vessels before transportation (CardioHelp, Maquet, New Jersey, USA).
Study design
The survival data were retrospectively analyzed using the statistical software, SPSS (IBM Corporation). Data are given as mean values ± standard deviation. Survival probabilities were analyzed using the Kaplan-Meier method. Differences in survival between groups were compared using the log-rank test. A null hypothesis probability value of less than 0.05 was considered statistically significant.
Results
Between January 2012 and October 2018, our mobile ECLS team has been sent out to evaluate 141 CS-patients in 39 peripheral hospitals and all received acute MCS. The mean distance to the peripheral hospitals was 83±86 km. Seven patients could not be stabilized by ECLS and died without being transported. The other 134 patients could be effectively stabilized by ECLS and were transported to our center (designated as ECLS patients in the following part of the manuscript). ECLS patients had a mean age of 53±13 years and 36 (27%) were female. Forty-three (32%) ECLS patients died on the system due to multi-organ failure, sepsis or severe cerebrovascular events. Twenty-nine (22%) ECLS patients were bridged to implantation of a durable MCS. Devices used were the HeartWare® in n=19, the TAH Syncardia® in n=2, the Berlin Heart Excor® BVAD in n=1, and the Thoratec BVAD® in n=4 patients. In 3 patients, cannulas were implanted as preparation for later pulsatile biventricular support and connected to two centrifugal pumps (Centrimag®). However, these 3 patients died. Two HeartWare®, 1 Syncardia® and 3 Thoratec BVAD® patients were bridged to transplantation. No patient was bridged to transplant on the ECLS system.
Fifty-nine ECLS patients could be weaned from the acute MCS system. There was a weaning failure with in-hospital mortality in 4 of these weaned ECLS patients, while 55 weaned ECLS patients could be discharged from our center. Twenty-seven of the weaned and discharged ECLS patients survived up to 1 year. Two patients were lost to follow-up, which was valued as death before 1-year.
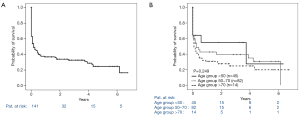
Overall, 1-year survival from the total number of 134 retrieved ECLS patients was 33% (n=44), including 11 patients alive on durable MCS and 2 patients alive after heart transplantation (Figure 1A). Interestingly, advanced age appeared not to be related to poor survival (Figure 1B).
Discussion
ECLS is not yet available in peripheral hospitals in Germany, because its usage requires experienced and regularly trained staff (4,8). Our mobile ECLS team consists of the regular staff in one of the largest, tertiary care cardiovascular centers in Germany, dealing with acute and durable MCS on a daily basis. The required hardware is readily available. Upon request, our team approach involves an experienced intensivist, an intensive care nurse and a pump-technician. The ECLS system carried along is the CardioHelp System (Maquet, New Jersey, USA), which is the only system approved for air travel (4). The mobile ECLS team may be sent out by helicopter or intensive care ambulance. Thus, tremendous efforts are made in terms of material, human resources and logistics (4,8). In view of the relatively sobering mid-term outcome after one year, it may be discussed, whether such enormous efforts are justified and how the prospective benefit of a CS-patient rescued by acute MCS implantation may be predicted more accurately.
We have herein presented anamnestic parameters prompted to the requesting center in order to at least roughly evaluate the CS-patients condition and potential for recovery under maximum therapy. There are no clear-cut limits for distinct requested items and decision making is not following a certain checklist, but is rather an overall impression with various attributes. Our data for example show that advanced age may not per se preclude the dispatch of the mobile ECLS team, as there was no relevant survival differences between the different age groups. However, this presumption is probably biased by the fact that other anamnestic parameters may have been weighed more extensively in the elderly patients. In fact, others noted advanced age adversely affects survival (9).
The herein presented outcome after durable MCS implantation is relatively poor. However, it should be noted that the ECLS patients were obviously all in an inter-agency registry for mechanically assisted circulatory support (INTERMACS) level 1 and these severely ill patients are known to have limited prognosis (10). Nevertheless, it is interesting to observe an equally poor outcome of the few ECLS patients transplanted subsequent to durable MCS implantation. In fact, all transplanted patients have been evaluated by our center’s multi-disciplinary transplant conference and they were approved for listing. Despite the fact that the very few numbers of finally transplanted ECLS patients herein may not be representative, it is a matter of debate when ECLS patients in INTERMACS level 1 and bridged with a durable MCS should be listed for heart transplantation.
In summary, we present our survival data for CS-patients retrieved under ECLS to our tertiary care center. The enormous efforts of establishing a mobile ECLS team are rewarded by survival of one third of these moribund patients. The relatively poor survival demonstrates the urgent need to define risk factors for morbidity and mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Patel H, Nazeer H, Yager N, et al. Cardiogenic Shock: Recent Developments and Significant Knowledge Gaps. Curr Treat Options Cardiovasc Med 2018;20:15. [Crossref] [PubMed]
- van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017;136:e232-68. [Crossref] [PubMed]
- Harjola VP, Mullens W, Banaszewski M, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:821-36. [Crossref] [PubMed]
- Guenther SPW, Buchholz S, Born F, et al. Remote ECLS-Implantation and Transport for Retrieval of Cardiogenic Shock Patients. Air Med J 2017;36:320-6. [Crossref] [PubMed]
- Shekar K, Mullany DV, Thomson B, et al. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 2014;18:219. [Crossref] [PubMed]
- Fukuhara S, Takeda K, Kurlansky PA, et al. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J Thorac Cardiovasc Surg 2018;155:1607-18.e6. [Crossref] [PubMed]
- Barge-Caballero E, Almenar-Bonet L, Gonzalez-Vilchez F, et al. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: a nationwide Spanish registry. Eur J Heart Fail 2018;20:178-86. [Crossref] [PubMed]
- Aubin H, Petrov G, Dalyanoglu H, et al. Four-year experience of providing mobile extracorporeal life support to out-of-center patients within a suprainstitutional network-Outcome of 160 consecutively treated patients. Resuscitation 2017;121:151-7. [Crossref] [PubMed]
- de Waha S, Graf T, Desch S, et al. Outcome of elderly undergoing extracorporeal life support in refractory cardiogenic shock. Clin Res Cardiol 2017;106:379-85. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]






