Early experience with Millipede IRIS transcatheter mitral annuloplasty
Abstract
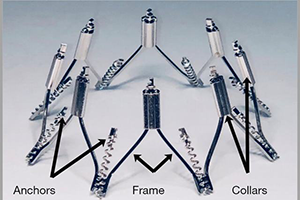
The IRIS mitral annuloplasty ring is a transcatheter, transfemoral and transseptal-delivered complete, semi-rigid annuloplasty ring. The IRIS system mimics surgical annuloplasty by reducing the mitral septal-lateral dimension and improving leaflet coaptation. We report the early experience with the IRIS system in seven patients. These patients had 3–4+ mitral regurgitation (MR) with annular dilation and were symptomatic NYHA II-IV with LV end systolic dimensions ≤65 mm. Patients were excluded for LVEF <20%, aortic valve disease, right-sided heart failure and PA systolic pressure >70 mmHg. Baseline and 30-day transthoracic echocardiography and CT imaging was performed. In phase 1, 4 patients had surgical IRIS mitral ring implantation. In phase 2, 3 patients had transfemoral, transseptal delivery of the IRIS mitral ring. There was no procedural death, or MI. The mitral SL diameter was reduced from 38.0±4.1 to 25.9±4.9 mm at 30 days (31.8% SL reduction, n=7). MR was reduced from baseline 3–4+ to 0–1+ in all patients at 30 days. There were improvements in NYHA class and there was a decrease in diastolic LV volumes from 182.4±54.3 to 115.3±98.8 mL at 30 days (36.8% reduction). Based on these initial positive findings, ongoing clinical trials are underway to further evaluate the safety and efficacy of the IRIS ring.
Cover