Early experience with the Intrepid system for transcatheter mitral valve replacement
Introduction
Severe mitral regurgitation (MR) is common and results in chronic volume overload, with a poor prognosis in patients with either symptoms or severe ventricular dilatation (1-3). Traditionally, MR necessitated surgical repair or replacement, however, new transcatheter methods have recently emerged. These methods offer a relatively less invasive alternative to open surgery and may help address an unmet clinical need for MR. In this report, we describe the methodology and present outcomes with the Medtronic IntrepidTM system for transcatheter mitral valve replacement (TMVR) (4).
The IntrepidTM TMVR system
The IntrepidTM TMVR system is a self-expanding, tri-leaflet bovine pericardial prosthesis housed within a nitinol frame. The frame consists of two parts: a circular outer fixation frame that comes in three sizes (43, 46, or 50 mm diameter) and a 27-mm circular inner stent frame (27 mm) (Figure 1). Implantation and fixation for anchoring is self-centering and symmetrical. The outer frame engages the dynamic native mitral valve anatomy with a flexible atrial brim that facilitates visualization under ultrasound during implantation. Fixation of the IntrepidTM prosthesis is achieved through oversizing and design features of the outer frame that allow the prosthesis to wedge itself in the sub-annular mitral space. The atrial portion of the outer frame is relatively flexible and conforms to the native annulus, while the ventricular portion is relatively stiffer. Small cleats on the outer frame also act as frictional elements to engage the native mitral leaflets. The dual frame structure allows the inner frame to remain circular throughout the cardiac cycle, without intrusion from the shape and motion of the outer frame and native annulus. There is no need for rotational orientation during device implantation. Risk of left ventricular outflow tract (LVOT) obstruction is minimized with a device profile of 17 to 18 mm. A hydraulically actuated delivery catheter is used to deliver the IntrepidTM with a 35 French (Fr) access sheath through the apex of the left ventricle, although, a transfemoral, transseptal version of the prosthesis is currently under development.

Implantation procedure
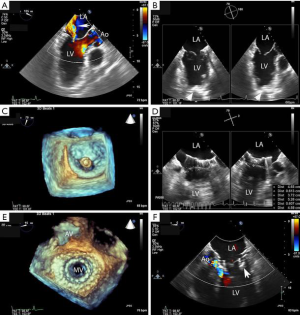
Patients with symptomatic primary or secondary MR may be considered for TMVR with the IntrepidTM system. All patients undergo contrast-enhanced, cardiac computed tomography (CT) of the mitral valve annulus to determine suitability and choice of the prosthesis size (6,7). In general, a prosthesis size is chosen that will allow 10% to 30% oversizing in the mitral annular perimeter, inter-commissural diameter and septal-lateral diameter, while minimizing risk of LVOT obstruction. While there are no set boundaries for neo-LVOT area, a predicted value of >1.3 cm2 is typically used. The cardiac CT scan is used to determine the access site for the thoracotomy and the location for placement of the sheath in the left ventricle. Patients are placed under general anesthesia for the procedure, with guidance by transesophageal echocardiography (TEE) and fluoroscopy (Figure 2). A small left thoracotomy is performed, followed by placement of left ventricular apical purse string sutures. A 6 or 7 French (Fr) vascular sheath is introduced into the left ventricle over a wire, followed by exchange for the IntrepidTM delivery catheter. The IntrepidTM catheter is directed to the left atrium with the assistance of TEE and centered in the mitral valve orifice. The atrial brim is expanded using hydraulic delivery, then aligned on the mitral annulus with care to maintain the brim in the left atrium. The IntrepidTM valve is deployed during a short run of rapid ventricular pacing. The delivery catheter is then withdrawn from the left ventricle and the apical access site is closed. In general, patients are hospitalized in the intensive care unit for 24 to 48 hours, followed by transfer to telemetry. Patients are placed on warfarin with international normalized ratio (INR) target range of 2.5 to 3.5 for three months, as well as a single antiplatelet agent, consisting of either aspirin (75, 81, or 100 mg daily) or clopidogrel (75 mg daily).
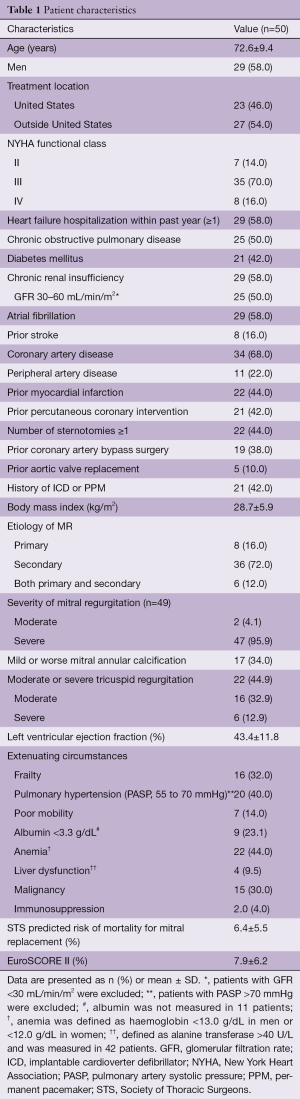
Patient outcomes
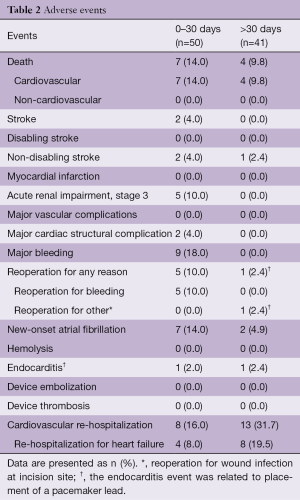
In 2015, a global feasibility study of the Medtronic IntrepidTM system was initiated with recruitment from 14 hospitals in Australia, Europe, and the United States (5). For this study, patients with symptomatic, severe MR who were at high or prohibitive surgical risk were considered for enrolment, with primary exclusion criteria being an ejection fraction <20%, mitral valve calcification, hemodynamic instability, severe pulmonary hypertension, severe renal insufficiency and prior mitral valve surgery or intervention. The results of the initial 50 patients (mean age, 72.6±9.4 years; 58% men; Table 1) who were consecutively enrolled through July 2017 have been published. Severe heart failure was common, with NYHA III or IV symptoms present in 86%. The predominant mechanism of MR was secondary (84% of patients). The mean left ventricular ejection fraction at baseline was 43.4%±11.8% (range, 20–70%). Overall, the population was at significantly increased risk of open surgery with an average STS-PROM of 6.4%±5.5% and a EuroSCORE II of 7.9%±6.2%. Successful implantation of the Intrepid valve occurred in 48 of 49 attempted patients (98.0%), with a median procedure time of 100 minutes [interquartile range (IQR), 80, 124] and a median time for device deployment of only 14 minutes (IQR, 12, 17). One malpositioning of the Intrepid valve occurred. There were no incidences of device malfunction or LVOT obstruction and no patient required conversion to open cardiac surgery. Seven deaths occurred within 30 days, with three as a result of apical access site bleeding, three as a result of refractory heart failure and one in the patient with malposition (Table 2).
Full table
Full table
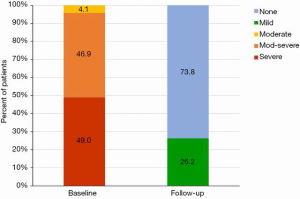
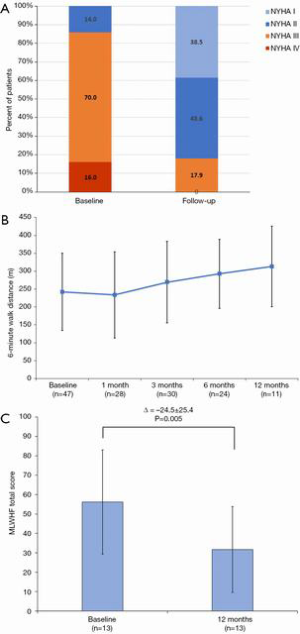
At a median follow-up of 173 (IQR, 54, 342) days, there were four additional deaths after 30 days (between day 54 and 122) but no deaths after 4 months (Figures 3,4); three of these late deaths were due to sudden cardiac arrest and one due to intracranial haemorrhage in the setting of an unwitnessed fall. Overall, the one-year survival rate was 77%. Mild para-prosthetic MR was present in three patients (7.1%), while mild prosthetic MR occurred in eight patients (19.0%) (Figure 5). For the survivors, mild or no symptoms of heart failure (i.e., NYHA functional class I or II) were present in 79% (P<0.0001 vs. baseline), and, for a subset of patients, there were significant improvements in the Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores (56.2±26.8 vs. 31.7±22.1; P=0.011) and six-minute walk distance (Figure 6). Pulmonary artery systolic pressure on transthoracic echocardiography decreased from 46.7±14.7 to 37.2±9.7 mmHg at follow-up (P<0.0001). No valve degeneration occurred and there were no instances of hemolysis, device embolization or thrombosis.

The APOLLO clinical trial
A multicenter, pivotal clinical trial was initiated in the fall of 2017 to examine the effectiveness and safety of the IntrepidTM System in patients with severe, symptomatic MR. The trial consists of two arms, with one-arm randomizing TMVR with vs. traditional surgery (n=650), and a second arm that will treat patients who are ineligible for surgery as a single cohort (n=550). For patients in the randomized cohort, the predicted risk of operative mortality is ≥3% with a combined <35% risk of mortality or irreversible major morbidity at 30 days, and patients must have an estimated life expectancy of >24 months. For the single-arm cohort, the predicted risk of operative mortality or irreversible major morbidity is ≥35% to 50% at 30 days and patients must have an estimated life expectancy of >12 months. The primary endpoint for the trial is a composite of all-cause mortality, stroke, re-operation (or re-intervention) and cardiovascular hospitalization at one year. Secondary safety endpoints include disabling stroke, acute kidney injury, prolonged ventilation, deep wound infection and major bleeding, while efficacy endpoints include the degree of MR improvement, change in quality of life, NYHA class and number of days alive out of hospital at one year.
Conclusions
Feasibility studies have demonstrated excellent results with the Intrepid system, with over 95% of patients successfully treated, a minimal learning curve, no device malfunction and improvements in symptom status and quality-of-life among the survivors. For all patients, meticulous attention to apical access techniques and post-operative heart failure care is essential. Ongoing clinical trials will determine the potential role of the Intrepid system compared with surgery and other transcatheter technologies in patients with MR.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [Crossref] [PubMed]
- Grigioni F, Detaint D, Avierinos JF, et al. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol 2005;45:260-7. [Crossref] [PubMed]
- Meredith I, Bapat V, Morriss J, et al. Intrepid transcatheter mitral valve replacement system: technical and product description. EuroIntervention 2016;12:Y78-80. [Crossref] [PubMed]
- Bapat V, Rajagopal V, Meduri C, et al. Early Experience With New Transcatheter Mitral Valve Replacement. J Am Coll Cardiol 2018;71:12-21. [Crossref] [PubMed]
- Blanke P, Dvir D, Cheung A, et al. Mitral Annular Evaluation With CT in the Context of Transcatheter Mitral Valve Replacement. JACC Cardiovasc Imaging 2015;8:612-5. [Crossref] [PubMed]
- Blanke P, Naoum C, Dvir D, et al. Predicting LVOT Obstruction in Transcatheter Mitral Valve Implantation: Concept of the Neo-LVOT. JACC Cardiovasc Imaging 2017;10:482-5. [Crossref] [PubMed]






