Medical management of aortic disease in Marfan syndrome
Introduction
Marfan syndrome (MFS) is a highly penetrant autosomal dominant disease with variable expressivity that often presents as dysfunction in a variety of different organ systems (1). One of the most vital structures affected by MFS is the cardiovascular system. The mutation in the fibrillin-1 gene (FBN1) gives rise to smooth muscle cell contractile dysfunction and a reduction in tensile strength of aortic tissue, thereby rendering the aorta maladapted/unfit to withstand the high pressures normally generated by the heart (1). Dysregulation of the transforming growth factor-β (TGF-β) pathway by FBN1 mutations has been shown to be a critical feature in aortic aneurysm development in MFS patients (2). The major cardiovascular manifestation of this microfibrillar disarray is best seen in the ascending aorta, where there is progressive aneurysmal dilatation that can bring about dissection or rupture (2). These dreaded complications are associated with a high mortality and represent majority of the premature deaths in MFS (3).
Along with early-onset aortic aneurysmal dilatation, there are other cardiovascular manifestations associated with this affliction, including mitral valve prolapse and left ventricular dysfunction (3). A very thorough clinical examination, complete family history and genetic analysis lead to a definitive diagnosis of MFS. Although there may be significant overlap in presentation with other connective tissue disorders, there is a precise criterion, known as the Ghent nosology (Table 1), available to diagnose this condition (4).
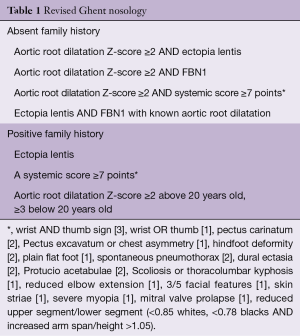
Full table
At the time of diagnosis, patients must undergo echocardiography to record baseline cardiovascular parameters (5). Echocardiogram should be supplemented with a computed tomography (CT) or magnetic resonance imaging (MRI) scan for cross-sectional aortic measurements, thus minimizing any chance of underestimating the true aortic size (5). Yearly follow up of aortic measurements is recommended; however, patients with a cross sectional diameter of >4.5 cm on the initial scan or a growth rate of ≥0.5 cm/year require more frequent (6 monthly) measurements (4). Aortic size may be reported as Z-scores, a useful tool that correlates aortic size with the body surface area (BSA) of patients (4). However, Z-score has its limitations. Uncertainty arises due to the multitude of different equations used for calculating Z-scores along with broad validation for Z-score nomograms and the uncertain natural history of aortic Z-scores in adult patients (6). Z-score also needs further validation in the pediatric population, as their constant growth impedes the proper determination of aortic size that is disproportionate to other body dimensions.
MFS patients require a multidisciplinary approach to management of cardiovascular and aortic manifestations. It is recognized that MFS patients with aortic diameters ≥5 cm in the ascending aorta and ≥6 cm in the descending aorta qualify for an open surgical repair (7), with surgery being far superior to endovascular stenting due to the elastic fragility of their vasculature (8). Pregnancy contributes additional risk due to hyper-dynamic circulation, therefore an aortic root diameter ≥4.5 cm is recognized for prophylactic aortic replacement prior to conception (9). Surgery remains the definitive treatment however, and medical therapy to halt the progression of aneurysm growth remains a matter of debate and ongoing research. In this review, we summarize the current knowledge regarding the medical management of patients with MFS.
Medical management in MFS
Current clinical studies have elucidated an optimal medical regimen for patients with MFS that may control the progression of cardiovascular manifestations and reduce the mortality associated with them (5,10-14). The standard of care for medical management constitutes the use of β-blockers with supplementation or replacement by angiotensin receptor blockers (ARBs), although this is a subject of ongoing research (5). Conflicting evidence exists amongst various studies as to which of these drugs might afford the best treatment of aortic disease; Extensive randomized controlled trials comparing various drug categories must be undertaken to better elucidate the effect of these medications on the cardiovascular and aortic manifestations of MFS.
β-blockers
It was shown in the 1970’s that decreasing the pressure impulse (dP/dt) limits the propagation of aortic dissection in a Tygon model and animal aortas (15). This led to the clinical application of β-blockers to decrease dP/dt. β-blockers exert both negative inotropic and chronotropic effects on the heart thereby effectively reducing the shear forces and blunting the maximal impulse produced by each systole (16). Literature demonstrates that long term (≥26 months) β-blocker therapy leads to an increase in the compliance of the aortic tissue in a subset of patients whose end-diastolic aortic diameter is <40 mm at the time of initiating these medications (10). Shores et al. (11) first demonstrated the benefit of beta blockade in Marfan patients in 1994. The mean dosage of 212±68 mg daily resulted in a significant decrease in the rate of change of aortic ratio (measured aortic diameter divided by predicted aortic diameter according to patient’s height, weight and age) from 0.084 to 0.023 per year in the untreated vs. treated group respectively (P<0.001) (11). Subsequent studies described the effect of beta blocker therapy in optimizing medical management in MFS patients (Table 2). The latest American College of Cardiology/American Heart Association (ACC/AHA) guidelines consider beta-blockers as the standard of care for adult patients with MFS and consider ARBs as an alternative (5). There is however a dearth of evidence regarding the optimal medical treatment for cardiovascular risk factors revolving around this condition in children.
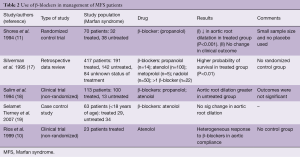
Full table
Despite being the standard of care for patients with MFS, the evidence that is available for β-blockers is chiefly derived from non-randomized controlled trials or studies with small sample sizes due to the relative rarity of this condition. A recent meta-analysis found that the data in favor of β-blocker therapy is insignificant or conflicted (12). Six out of sixteen studies demonstrating the effects of β-blockers in MFS were scrutinized and only one of them projected a beneficial effect in terms of odds ratio for mortality and adverse events (OR =0.6, 95% CI =0.18–2.01) (5,10,11,15). Furthermore, there are numerous side effects associated with the long-term use of beta-blocker therapy which may include bradyarrhythmia, bronchospasm in patients with asthma/chronic obstructive pulmonary disease (COPD), sexual dysfunction, mood disturbance and masking of reflex sympathetic symptoms in patients with diabetes (12). These affects may add morbidity and adversely impact quality of life in patients who require long term treatment, such as those with MFS.
ARBs and angiotensin converting enzyme inhibitors (ACEIs)
The renin-angiotensin-aldosterone system (RAAS) closely interplays with other biochemical pathways in the body to produce regulatory effects on the cardiovascular system. It has been found that angiotensin II contributes to endothelial cell hypertrophy and proliferation via the NADH/NADPH oxidase system (7). Furthermore, angiotensin II promotes the formation of TGF-β resulting in activation of various matrix metalloproteinases (MMP) that degrade the medial layers of vessels such as the aorta (20). These catabolic reactions weaken the fragile vascular tissue of MFS patients, paving way for complications such as aneurysm, dissection and rupture (20).
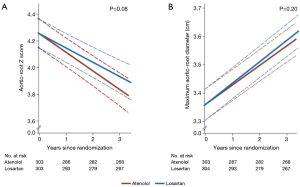
Due to their mode of action (antagonizing pathophysiological mechanisms) ARBs and ACEIs are promising therapies for patients with MFS. Nevertheless, there has been a constant debate surrounding the efficacy of ARBs compared to ACEIs, with both animal and human studies favoring the use of ARB (20). Losartan (ARB) has been found to limit the signaling effects of TGF-β, thereby reducing the damage caused by matrix degradation in the aortic wall (21). In 2008, shortly after Habashi et al. (20) demonstrated that TGF-β was associated with aneurysm formation in mice, a cohort of 17 pediatric MFS patients were treated with losartan (22); follow up with serial echocardiograms demonstrated that the rate of increase in the aortic root diameter after ARB therapy was reduced amongst all patients compared to those untreated (3.54 vs. 0.46 mm/yr) (P<0.001) (22). In 2014, a RCT was employed to demonstrate the difference between atenolol and losartan (the mean dosages prescribed were 151±75 and 85±14 mg daily, respectively) (23). Results of this RCT did not demonstrate any statistical difference between the two drugs in terms of aortic root Z-scores followed for 3 years (−0.139 vs. −0.107 respectively, P=0.08); Additionally, there was no statistically significant difference in the 3-year rates of aortic surgery, dissection, or death amongst the two groups described (23). This study has been interpreted differently by various experts. Some say this study finds ARBs equally effective as β-blockers. Others, pointing out the justifiable doubt regarding the fundamental benefit of β-blockers, interpret this study as showing that ARBs are essentially equivalent to a placebo (24).
Yetman et al. compared β-blockers with ACEIs in MFS patients (25). This study was based on evidence that ACE inhibitors help to reduce the vascular smooth cell apoptosis prevalent in MFS patients, thereby protecting from aortic dissection (26). Interestingly, the results showed that the group prescribed with ACEI had relative conservation of the elastic aorta as compared to patients who were on β-blockers (25). This was indicated by an aortic stiffness index for the enalapril group vs. propanolol/atenolol group, which measured 8±2.9 vs. 18.4±3.8 (P<0.05) respectively (25). Likewise, aortic distensibility was found to be favorable in the enalapril (ACEI) group compared to the propanolol/atenolol (B-blocker) group, 3±0.3 vs. 1.9±0.4 cm2·dynes−1 respectively (P<0.02) (25).
To further elucidate the role of ACEIs, Williams et al. did a cross-over study using three classes of drugs, including ACEIs, β-blockers and calcium channel blockers (CCBs) (13). The results did not reveal any significant difference amongst the various treatment groups (after 4 weeks) with regards to variation of blood pressure (13). There was also no significant difference in hemodynamic effects from these medications. However, the time interval from cardiac systole to peak systolic dilatation of the aorta was prolonged in the atenolol group (β-blocker) for both the aortic arch (increase of 8%) and abdominal aorta (increase of 11%) compared to the ACEI group and CCBs (P<0.01, 0.05 respectively) (13).
MFS patients are prone to develop aneurysmal dilatation, specifically of the aortic root where, ARBs have shown a promising role in halting its progression (22). Summarizing the use of ARBs, it is safe to say that ARBs are emerging as an equally effective, if not better, alternative to β-blockers in MFS syndrome patients (14) (Table 3).
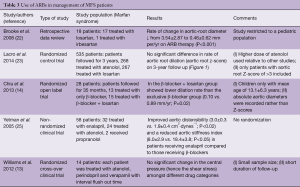
Full table
Statins
Statins inhibit the rate limiting step of cholesterol synthesis by blocking the HMG-CoA reductase enzyme, thereby reducing the formation of cholesterol precursors. By decreasing lipid formation, they also help in reducing the pro-inflammatory mediators known to be involved in aneurysm formation such as protein kinase-c (PKC) and TGF-β (27). In vivo experiments demonstrated that statins play a role in reducing the expression of cardiac TGF-β in mice (27). Furthermore, the same study demonstrated that the statin group was found to have higher expression of endothelial NO synthase (eNOS) than placebo (27). eNOS is known to generate vasodilatory mediators that are beneficial in reducing the sheer stress (dP/dt) within the vasculature.
An animal study comprising of Marfan mice treated with statin (pravastatin) or losartan compared with a control group demonstrated a clear decline in aortic root diameter enlargement from 0.252 cm in the untreated group to 0.22 and 0.221 cm in the statin and ARB groups, respectively (P≤0.01 for both) (28) The study further demonstrated that losartan was better than pravastatin in preserving the elastic component of the aortic media and maintaining a lower pulse pressure compared to other groups (28).
Interestingly the aortic medial layer thickness was irregular in Marfan mice (152±13 µm) in comparison to normal mice (104±14 µm) (P<0.01) (28). The group treated with losartan demonstrated significant normalization of the medial layer in Marfan mice (112±13 µm), whereas pravastatin did not show any significant effect on medial layer thickness (28).
Our group has previously demonstrated the protective role of statins in a retrospective study of 1,560 patients with thoracic aortic aneurysms of all varieties who were treated with statins (369 patients) compared to those who did not receive them (1,191 patients) from the year 1985 to 2011. Our study concluded that statins reduce the yearly rate of dissection, rupture and death in patients with all types of aortic aneurysms, except those limited exclusively to the aortic root (P=0.001–0.01) (29). Moreover, statin therapy also increased the interval from diagnosis to adverse event or surgery (29). Statin therapy also reduced the total number of patients who eventually required surgery 58% (untreated) vs. 48% (statin) (P<0.018) (29). These findings highlight the possibility of a role of statins in long term treatment of MFS patients. However, it is important to remember that statins have not shown significant protective effects on the aortic root (most commonly involved in MFS) and further prospective randomized controlled trials are needed.
CCBs
CCBs have been used as antihypertensive agents, especially in the African-American population that demonstrates resistance to ACEI and ARBs (30). Initially CCBs were proposed to have similar therapeutic benefits in MFS as other anti-hypertensive according to Rossi-Foulkes et al. in 1999 (31): twenty patients were given propranolol, six patients received a calcium channel antagonist and 27 patients continued without any medication. They were followed for 44±24 months. This study demonstrated a significant difference in the aortic growth rate, 1.8±0.9 vs. 0.9±1.3 mm/year in untreated and treated patients respectively (P<0.02) (31). The study was limited, however by the very small sample size and multiple cross overs from β-blocker group to the CCB group. Furthermore, the results measured outcomes inclusive of both medications rather than separately accounting for CCBs therefore the results might not be truly representative of CCB effects.
Recent animal studies demonstrated a detrimental effect of CCB on Marfan mice, as mice treated with CCBs exhibited higher rates of dissection on 3-month follow-up compared to those given placebo (32). This discovery was also found to have relevance in humans, as retrospective trials of CCB agents aiming to evaluate adverse outcomes displayed similar results. Marfan patients who received CCB (n=531) prior to or at the time of their diagnosis (followed up for a mean time of 50.8±1.6 months), when compared to groups not taking CCBs, were found more likely to have acute aortic dissections [odds ratio (OR) =12.5; P=0.032] (32). Patients on CCBs had greater odds of having surgery than those on other hypertensive regimens (OR =5.5, P=0.001) (32). Findings from this study revealed that patients on amlodipine had worse outcomes than those taking verapamil, which may be explained by the known selectivity of verapamil for cardiac tissue (32). CCBs are hypothesized to cause this damage via activation of the TGF-β dependent signaling cascades (32). Further research is required to define the optimal anti-hypertensive regimen for MFS. Nevertheless, current evidence suggests that CCBs are not a first-line choice.
Current practice and future perspectives
Based on current evidence, there is a definite role for prophylactic medical management of Marfan patients at the time of diagnosis. Nonetheless, the true effectiveness is questionable, and the choice of most effective medication along with its ideal dosage regimen remains to be elucidated. Currently, β-blockers are the preferred method of management, with ARBs emerging as an equally effective strategy. B-blockers, ARBs and statins when combined, may potentially have an additive beneficial effect on decreasing the rate of progression of aortic aneurysms, although this theory needs further evaluation (14). While all MFS patients have a predisposition to develop thoracic aortic aneurysm and dissection, the decision to initiate long-term medical management with anti-hypertensives should be individualized to the patient, as benefit is unproven. Not only are further studies needed in adults, but also it is important to conduct RCTs comparing various drug therapies in children, as modern diagnostic modalities now enable a large proportion of patients with MFS to be diagnosed at an earlier age.
Conclusions
Medical management of patients with MFS is considered to play a pivotal role in the over-all care offered to these patients, although the precise effectiveness is yet to be discovered. ARBs have shown great potential in vitro, targeting most biochemical pathways leading to aneurysm formation. Additionally, ARBs may have a comparatively better side-effect profile than β-blockers. Clinical studies of ARBs, however, have been inconclusive. Further research with RCTs is still required, to help establish the relative effectiveness of various medications used in patients with MFS. It may be that the failure of observational and randomized studies to show distinct benefits of any drug (or superiority of a particular class) arises because the beneficial effects are not real.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Milewicz DM, Michael K, Fisher N, et al. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation 1996;94:2708-11. [Crossref] [PubMed]
- Franken R, den Hartog AW, Radonic T, et al. Beneficial Outcome of Losartan Therapy Depends on Type of FBN1 Mutation in Marfan Syndrome. Circ Cardiovasc Genet 2015;8:383-8. [Crossref] [PubMed]
- De Backer J. Cardiovascular characteristics in Marfan syndrome and their relation to the genotype. Verh K Acad Geneeskd Belg 2009;71:335-71. [PubMed]
- Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476-85. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-e369. [Crossref] [PubMed]
- Curtis AE, Smith TA, Ziganshin BA, et al. The Mystery of the Z-Score. Aorta (Stamford) 2016;4:124-30. [Crossref] [PubMed]
- Danyi P, Elefteriades JA, Jovin IS. Medical therapy of thoracic aortic aneurysms: are we there yet? Circulation 2011;124:1469-76. [Crossref] [PubMed]
- Nordon IM, Hinchliffe RJ, Holt PJ, et al. Endovascular management of chronic aortic dissection in patients with Marfan syndrome. J Vasc Surg 2009;50:987-91. [Crossref] [PubMed]
- European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM), et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147-97. [Crossref] [PubMed]
- Rios AS, Silber EN, Bavishi N, et al. Effect of long-term beta-blockade on aortic root compliance in patients with Marfan syndrome. Am Heart J 1999;137:1057-61. [Crossref] [PubMed]
- Shores J, Berger KR, Murphy EA, et al. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan's syndrome. N Engl J Med 1994;330:1335-41. [Crossref] [PubMed]
- Gersony DR, McClaughlin MA, Jin Z, et al. The effect of beta-blocker therapy on clinical outcome in patients with Marfan's syndrome: a meta-analysis. Int J Cardiol 2007;114:303-8. [Crossref] [PubMed]
- Williams A, Kenny D, Wilson D, et al. Effects of atenolol, perindopril and verapamil on haemodynamic and vascular function in Marfan syndrome - a randomised, double-blind, crossover trial. Eur J Clin Invest 2012;42:891-9. [Crossref] [PubMed]
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to beta-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013;88:271-6. [Crossref] [PubMed]
- Prokop EK, Palmer RF, Wheat MW Jr. Hydrodynamic forces in dissecting aneurysms. In-vitro studies in a Tygon model and in dog aortas. Circ Res 1970;27:121-7. [Crossref] [PubMed]
- Feldman M, Shah M, Elefteriades JA. Medical management of acute type A aortic dissection. Ann Thorac Cardiovasc Surg 2009;15:286-93. [PubMed]
- Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol 1995;75:157-60. [Crossref] [PubMed]
- Salim MA, Alpert BS, Ward JC, et al. Effect of beta-adrenergic blockade on aortic root rate of dilation in the Marfan syndrome. Am J Cardiol 1994;74:629-33. [Crossref] [PubMed]
- Selamet Tierney ES, Feingold B, Printz BF, et al. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr 2007;150:77-82. [Crossref] [PubMed]
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312:117-21. [Crossref] [PubMed]
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013;34:3491-500. [Crossref] [PubMed]
- Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med 2008;358:2787-95. [Crossref] [PubMed]
- Lacro RV, Dietz HC, Sleeper LA, et al. Atenolol versus losartan in children and young adults with Marfan's syndrome. N Engl J Med 2014;371:2061-71. [Crossref] [PubMed]
- Ziganshin BA, Mukherjee SK, Elefteriades JA. Atenolol versus Losartan in Marfan's Syndrome. The New England journal of medicine 2015;372:977-8. [Crossref] [PubMed]
- Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol 2005;95:1125-7. [Crossref] [PubMed]
- Nagashima H, Sakomura Y, Aoka Y, et al. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan's syndrome. Circulation 2001;104:I282-7. [Crossref] [PubMed]
- Yu Y, Ohmori K, Chen Y, et al. Effects of pravastatin on progression of glucose intolerance and cardiovascular remodeling in a type II diabetes model. J Am Coll Cardiol 2004;44:904-13. [Crossref] [PubMed]
- McLoughlin D, McGuinness J, Byrne J, et al. Pravastatin reduces Marfan aortic dilation. Circulation 2011;124:S168-73. [Crossref] [PubMed]
- Stein LH, Berger J, Tranquilli M, et al. Effect of statin drugs on thoracic aortic aneurysms. Am J Cardiol 2013;112:1240-5. [Crossref] [PubMed]
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993;328:914-21. [Crossref] [PubMed]
- Rossi-Foulkes R, Roman MJ, Rosen SE, et al. Phenotypic features and impact of Beta blocker or calcium antagonist therapy on Aortic lumen size in the Marfan syndrome. Am J Cardiol 1999;83:1364-8. [Crossref] [PubMed]
- Doyle JJ, Doyle AJ, Wilson NK, et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife 2015;4:e08648. [Crossref] [PubMed]





