Surgical management of tricuspid stenosis
IntroductionOther Section
Anatomy
The tricuspid valve consists of three leaflets (anterior, posterior, and septal), their associated chordae tendineae and papillary muscles, the fibrous tricuspid annulus, and the right atrial and right ventricular myocardium. The anterior leaflet is quadrangular in shape and typically the largest of all leaflets. The posterior leaflet is notable for the presence of two or more scallops and is generally triangularly-shaped. The septal leaflet is small, attached to the right and left fibrous trigones and atrial and ventricular septa, and relatively immobile (Figure 1).

The triangle of Koch is defined by the annulus of the septal leaflet, the coronary sinus and the tendon of Todaro. The atrioventricular (AV) node is in the apex of this triangle, and the bundle of His runs from the AV node through the central fibrous body in the interventricular membranous septum (Figure 1). The membranous portion of the interventricular septum is closely approximated to the septal leaflet, as is the AV node. These relationships are important to consider during tricuspid valve replacement (TVR), and some authors advocate leaving a small ring of septal leaflet tissue in which to place sutures, thereby avoiding the conduction system and nodal blockade (Figure 2). The location of the AV node in relationship to this septal leaflet has also been accommodated in the design of annuloplasty rings, which have a gap in the ring in this area.
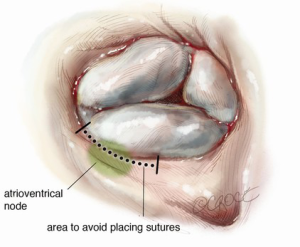
The anterior papillary muscle provides chordae to the anterior and posterior leaflets, the posterior papillary muscle provides chordae to the posterior and septal leaflets, and the septal wall gives chordae to the anterior and septal leaflets. Additionally, there may be accessory chordal attachments to the right ventricular free wall and to the moderator band. A range between two to six papillary muscles has been described in association with the tricuspid valve (1).
Pathophysiology of tricuspid stenosis (TS)
There are several etiologies of TS. Most commonly, TS results from rheumatic heart disease, and accounts for the majority of surgically excised stenotic tricuspid valves. It is commonly seen in association with mitral stenosis or with both aortic and mitral stenosis. Other less common causes of TS include congenital abnormalities (Ebstein’s anomaly; or isolated tricuspid valve stenosis); metabolic or enzymatic abnormalities (Fabry’s disease, Whipple’s disease, carcinoid); or active infective endocarditis (2).
The grading of TS severity is not generally agreed upon, but a mean gradient greater than 5 mm Hg at a normal heart rate is considered severe TS. This transtricuspid diastolic gradient is highly variable and is affected by heart rate, forward flow, and phases of the respiratory cycle. Thickened and distorted leaflets and a valve area of less than 1.0 cm2 helps further characterize TS. This is in contrast to a normal valve area of 4.0 cm2 (3).
TS is usually progressive when due to rheumatic disease or carcinoid, versus a fixed stenosis in the setting of congenital abnormalities. Moreover, most stenotic tricuspid valves have some element of tricuspid regurgitation. This is in contrast to purely regurgitant tricuspid valves, which have no element of TS. Stenotic tricuspid valves always demonstrate structural abnormalities, such as fibrous thickening of the leaflets or subvalvular mural plaque as seen in carcinoid. Each etiology of TS has its own distinct pattern of leaflet and chordal pathology (4).
Rheumatic tricuspid disease is characterized by diffuse fibrous thickening of the leaflets with fusion of two to three commissures. Interestingly, in contrast to mitral valve stenosis, tricuspid leaflet thickening is usually in the absence of calcific deposits, making it amenable to surgical or transluminal balloon valvuloplasty. However, when tricuspid leaflet fusion results in a fixed central orifice with accompanying TR, commissurotomy is not typically an option.
Carcinoid results in right-sided valvular lesions in about 50% of patients with metastatic disease. Carcinoid lesions are distinctive fibrous white plaques located on valvular or mural endocardium and result in thickened, rigid and foreshortened leaflets. In a study investigating patients who underwent TVR for carcinoid disease, progression of carcinoid plaques was noted on autopsy despite successful management of the primary tumor. This has led some authors to suggest that TVR in carcinoid patients should be managed with a mechanical valve to avoid degeneration of the bioprosthetic valve (5).
Congenital heart disease may cause TS by a variety of mechanisms. Incompletely developed leaflets, shortened or malformed chordae, a small annulus, or an abnormal number or size of papillary muscles may result in TS. Ebstein’s anomaly typically results in TR, but can also lead to TS in patients with leaflets that remained fused as a diaphragm across the valve orifice.
Other rare causes of TS include infective endocarditis, Fabry’s or Whipple’s disease, or methysergide exposure. Right atrial or ventricular tumors may also result in functional TS.
Indications for surgical management
The 2014 AHA/ACC Valvular Heart Disease Guidelines recommends tricuspid valve surgery for (A) patients with severe TS at the time of operation for left-sided valve disease and (B) isolated, symptomatic severe TS. These class I recommendations are supported by level C evidence. The guidelines further suggest percutaneous balloon commissurotomy in patients with isolated, symptomatic severe TS without accompanying TR (6).
The European Society of Cardiology (ESC) guidelines similarly recommend surgery for severe TS in patients undergoing concomitant cardiac surgery, or for severe TS in symptomatic patients despite medical optimization. Again, guidelines call for percutaneous balloon commissurotomy in patients with isolated TS, no TR, and without calcified tricuspid valves (3).
Operative techniqueOther Section
Preparation
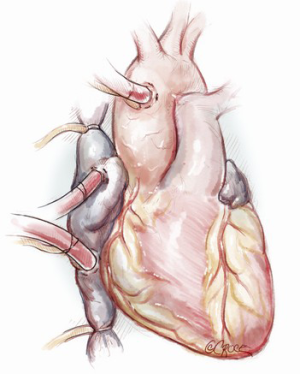
The patient is positioned supine on the operating room table. General anesthesia is induced, and a urinary catheter and arterial and venous lines are placed. A transesophageal echocardiography (TEE) probe may also be passed. Surgical exposure for the tricuspid valve is via a median sternotomy or a right thoracotomy, and choice of incision depends both on surgeon comfort and the presence of any additional pathology that needs correction. The setup for cardiopulmonary bypass is also dependent on indications for surgery and surgical exposure. When performing a conventional sternotomy for isolated TS, ascending aortic and bicaval cannulation is done. We typically place a superior vena cava (SVC) cannula into the right atrial appendage but it may also be placed directly into the SVC. For patients undergoing a right thoracotomy, peripheral venous cannulation may be performed via the right internal jugular and femoral veins. Arterial cannulation may be performed directly into the ascending aorta or via the axillary, femoral, or right subclavian artery (7). We favor a sternotomy approach, and typically cannulate the distal ascending aorta (Figure 3).
Tricuspid valve surgery may be performed with or without aortic cross-clamp. In beating heart surgery, TEE should be used to assess the presence of a patent foramen ovale (PFO) or atrial septal defect (ASD) prior to right atriotomy. These will need to be closed upon opening the atrium. Visual inspection and gentle probing of the fossa ovalis may also be performed to confirm the TEE findings. We also snare or clamp the SVC and inferior vena cava (IVC) to keep the field clear of blood.
Exposition
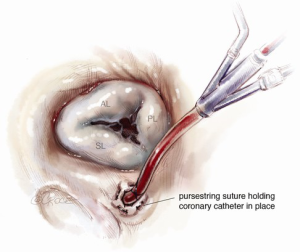
In tricuspid valve surgery performed on an arrested heart, we snare or clamp the SVC and IVC, and employ both antegrade and retrograde cardioplegia and an aortic root vent. To further clear the field of blood, we suggest placing a purse-string suture around the coronary sinus. The retrograde cardioplegia catheter is then placed into the coronary sinus and slowly withdrawn until only its most proximal portion is in the sinus. The purse-string is then cinched around the cardioplegia line. This will allow for greatest distribution of cardioplegia and also prevent reflux back into the field (Figure 4) (8).
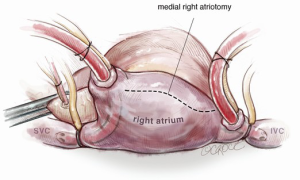
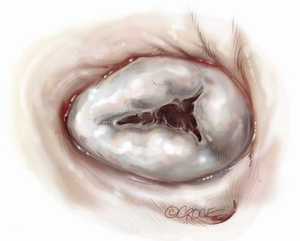
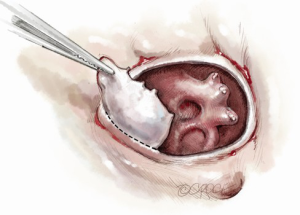
For isolated tricuspid procedures, we make a right atriotomy parallel to the AV groove (Figure 5). We generally start our atriotomy close to the appendage and extend it to just above the IVC, being careful to leave enough tissue for adequate tension-free closure. Pledgeted traction sutures are placed to facilitate exposure, and the valve is inspected to identify and confirm its underlying pathology. TS is commonly due to a structurally damaged valve that is beyond repair and requires excision of leaflets, and the lack of pliable leaflet tissue is the main limitation for valve repair (Figure 6). An attempt should be made to retain the subvalvular apparatus, similar to the approach taken in chordal-sparing mitral valve replacement (Figure 7). A small portion of the septal leaflet is also left intact to avoid injury to the AV node.
Operation
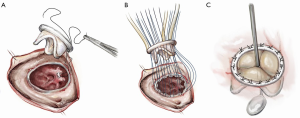
After the deformed leaflets are excised, the prosthetic valve is seated. For biologic valves, we use a supra-annular technique, suturing from the ventricular side to the atrial side and then through the sewing ring. The valve is seated, the sutures are tied and cut, and the right atrium is closed and de-aired. If a bioprosthetic valve is placed, the use of a small dental mirror to visualize the inside of the ventricle to confirm optimal placement is a useful adjunct (Figure 8). For mechanical valves, we use interrupted pledgeted horizontal mattress sutures with an everting technique, passing the suture from the atrial side through to the ventricle and then through the valve’s sewing ring (Figure 9).
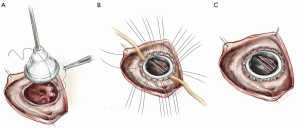
Broadly speaking, biological prostheses for TVR are preferred over mechanical ones because of the higher risk of thrombosis carried by the mechanical valve (9). A patient with a mechanical valve in the tricuspid position must be closely managed with an international normalized ratio (INR) between 2.5 to 3.5. These valves are at a higher risk of a thrombotic complication than mechanical valves in the aortic or mitral position, but patients may have more bleeding complications given the relatively higher INR. Biologic valves, on the other hand, have issues with long-term durability, and early degeneration is problematic in patients with carcinoid syndrome. However, these patients could be candidates for valve-in-valve transcatheter replacement in the future. Moreover, in certain patient populations, such as those with carcinoid, a mechanical valve may be more appropriate, given the potential for recurrence of carcinoid plaques. Homograft replacement of the tricuspid valve has been described, but these reports are from isolated or intermittent case reports and case series.
In our practice we nearly always replace the tricuspid valve. However, in cases of intractable infective endocarditis, some authors suggest complete excision of the valve without replacement. Arbulu et al. report on 55 patients who underwent tricuspid valvulectomy without replacement. Notably these patients were young, had normal pulmonary artery pressures, and were otherwise healthy. In this patient population, the absence of a tricuspid valve is well tolerated, with long-term mortality 64% at 22 years (10).
Completion
After the valve is satisfactorily seated, the retrograde coronary sinus catheter is removed and the atrium is closed in two layers. The caval snares are removed after the first layer is completed, and some blood is left in the heart in order to close the right atrium without undue tension. The patient is placed in steep Trendelenburg, and the aortic cross-clamp is removed. If appropriate care was taken during annular suture placement, the heart should resume normal sinus rhythm, and the patient is weaned off cardiopulmonary bypass. Routine decannulation and sternal closure ensues.
Alternatives
In the modern era, percutaneous balloon valvuloplasty is frequently used to slow or reverse an ongoing fibrotic process and increase leaflet mobility, and also used in patients who are not operative candidates or do not desire surgery. If open valvuloplasty is performed, it is typically in the setting of a patient with just one or two fused commissures. The anteroseptal commissure is most frequently fused.
Care must be taken to incise the commissure so that its two edges are each supported by chordae. In severe cases it may be difficult to precisely locate the commissure, but extra care must be taken to avoid injury to the small commissural “leaflets” that may be present. Thus, once an initial incision is made, the subvalvular apparatus must be inspected to further guide the incision into the papillary muscle by approximately 1 cm. This helps to re-establish leaflet mobility.
Transcatheter TVR is increasingly described in the literature. One case report describes the percutaneous placement of a tricuspid valve in a patient with two prior tricuspid valve repairs, the latter with a 32-mm Carpentier-Edwards annuloplasty ring for rheumatic disease. A 29-mm Sapien XT valve was placed with minimal paravalvular leak and her symptoms resolved (11). A case series, from Eicken and colleagues, describes percutaneous valve-in-valve replacement for bioprosthetic tricuspid valve stenosis. Most patients in this study had congenital abnormalities requiring TVR during childhood. Results were favorable, with the percutaneous valve functioning well in 15 out of 16 patients at 2 years follow-up (12). Both studies involved collaboration between interventional cardiologists and cardiac surgeons, and as the technology improves, we only expect the use of percutaneous valves to increase.
CommentsOther Section
Clinical results
Existing studies investigating outcomes after surgical TVR are limited. Most have small series of patients, or are dated and span a considerable length of time and therefore do not account for advancements made in the modern era of cardiac surgery and cardiopulmonary bypass. Reoperations are also included in the studies, further coloring outcomes. Patients included in the studies also have variable etiologies for undergoing TVR, making accurate comparisons difficult. Because of this, the mortality rates in these case series are higher than anticipated for modern isolated TVR. Nonetheless, it is important to review what the literature tells us about outcomes on TVR for stenosis.
Iscan and colleagues studied the long-term results of patients undergoing TV replacement. Forty-two patients over a period of 17 years (from 1987 to 2004) underwent replacement for tricuspid valve stenosis. Results showed an in-hospital mortality of 26% and a 10-year event free survival of 31%. Rheumatic etiology and elevated pulmonary artery pressures were risk factors for poor outcome (13). These results are not dissimilar from those reported by other groups. Poveda and associates conducted a retrospective analysis of the outcomes of 62 consecutive patients who underwent TVR for rheumatic disease between 1974 and 1994. Hospital mortality was 37% and was associated with elevated pulmonary artery pressures and poor clinical functional status (14).
Several other studies have also looked at the results of TVR with biologic or mechanical valves. The largest study was conducted by Ratnatunga and associates. Using the UK Heart Valve Registry, 425 patients were identified over a period of 20 years who underwent TVR. Two hundred and twenty five of these patients (53%) received a biologic valve and 200 (42%) received mechanical valves. Thirty-day mortality was 17% and 5-year survival was 60%, and there was no difference in outcomes between biologic or mechanical valves (15). Kaplan and colleagues also investigated this question. One hundred and twenty nine TVRs were performed over a period of 20 years. Bioprosthetic valves were used in 32 patients, whereas 97 patients had mechanical valve implantation. Thirty-day mortality was 24.5% and there was no statistically significant difference between the two groups in terms of early mortality, re-replacement, and midterm mortality. The authors go on to recommend low profile modern bileaflet mechanical valves for prosthetic replacement of the tricuspid valve. The favorable hemodynamic characteristics and durability of the low-profile mechanical valve outweighed the rare risks of prosthetic valve thrombosis and pulmonary embolism in 10 patients (16).
Caveats
There are several surgical pitfalls associated with replacing the tricuspid valve. Iatrogenic injury to the right coronary artery (RCA) has been described. The RCA runs in the AV groove and closest to the tricuspid valve in the area of the posterior leaflet. Injury to the artery can result from either placing undue tension on the adjacent tissue resulting in a functional stenosis, or by directly suturing the artery. If the complication is noted intra-operatively, release of the culprit stitch or immediate bypass should be performed. Post-operatively, catheter-based approaches have been described for functional stenoses.
Care should also be taken to not injure the AV node. When suturing along the septal leaflet in the region of the anteroseptal commissure, myocardial tissue should be avoided with sutures placed in the base of the leaflet. This minimizes the risk of AV nodal blockade. If performing beating heart TVR, a stitch too close to the AV node would result in rhythm disturbance. This immediate feedback allows the surgeon to re-do the stitch and avoid permanent injury to the node.
Another important consideration is if a concomitant aortic valve procedure is planned or a patient has a prior aortic valve replacement. The anteroseptal commissure of the tricuspid valve is very close to the noncoronary cusp of the aortic valve. If a stented aortic bioprosthesis is in place, anchoring sutures may be difficult to place. Furthermore, a poorly placed commissure suture may inadvertently injure or plicate open the non-coronary cusp, resulting in acute aortic insufficiency.
Recommendations and conclusions
The surgical management of tricuspid valve stenosis is an important but under-represented topic in contemporary cardiac surgery literature. The authors would like to emphasize several key points:
- Rheumatic and carcinoid disease are the most frequent causes of TS;
- Surgical pitfalls include injury to adjacent structures such as the RCA and the AV node, and care should be taken in patients who have undergone prior AVR;
- Outcomes studies are useful but should be interpreted with caution as most studies span one or more decades, and include a heterogeneous patient population including re-operative tricuspid surgery and differing etiology of tricuspid valve stenosis;
- There does not appear to be clear superiority in terms of replacement with a mechanical or biologic prosthesis. Mechanical valves are more prone to thrombosis, but biologic valves may fail slowly. Individual patient characteristics must be considered when choosing valve type;
- We expect the frequency of percutaneous TVR to increase in the future.
With our current trajectory of research and clinical care, we look forward to innovative surgical and interventional management and improved outcomes in the treatment of this rare pathology.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Ailawadi G. Tricuspid Valve. Mastery of Cardiothoracic Surgery. 3rd Edition. Wolters Kluwer 2013:779-86.
- Waller BF, Howard J, Fess S. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation—part I. Clin Cardiol 1995;18:167-74. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [Crossref] [PubMed]
- Blaustein AS, Ramanathan A. Tricuspid valve disease. Clinical evaluation, physiopathology, and management. Cardiol Clin 1998;16:551-72. [Crossref] [PubMed]
- Palaniswamy C, Frishman WH, Aronow WS. Carcinoid Heart Disease. Cardiol Rev 2012;20:167-76. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [Crossref] [PubMed]
- Shemin RJ. Tricuspid Valve Disease. In: Cohn LH. editor. Cardiac Surgery in the Adult. 4th Edition. NY: McGraw-Hill, 2012.
- Duran CG. Surgical Treatment of the Tricuspid Valve. In: Sellke F, del Nido PJ, Swanson SJ. editors. Surgery of the Chest. 8th Edition. PA: Saunders Elsevier, 2010:1241-55.
- Shinn SH, Schaff HV. Evidence-based surgical management of acquired tricuspid valve disease. Nat Rev Cardiol 2013;10:190-203. [Crossref] [PubMed]
- Arbulu A, Holmes RJ, Asfaw I. Tricuspid valvulectomy without replacement. Twenty years’ experience. J Thorac Cardiovasc Surg 1991;102:917-922. [PubMed]
- Bouleti C, Himbert D, Brochet E, et al. Transfemoral Tricuspid Valve-in-ring implantation using the Edwards Sapien XT valve. Circ Cardiovasc Interv 2015;8:e002225. [Crossref] [PubMed]
- Eicken A, Schubert S, Hager A, et al. Percutaneous Tricuspid Valve Implantation: Two-center experience with midterm results. Circ Cardiovasc Interv 2015;8:e002155. [Crossref] [PubMed]
- Iscan ZH, Vural KM, Bahar I, et al. What to expect after tricuspid valve replacement? Long-term results. Eur J Cardiothorac Surg 2007;32:296-300. [Crossref] [PubMed]
- Poveda JJ, Bernal JM, Matorras P, et al. Tricuspid valve replacement in rheumatic disease: preoperative predictors of hospital mortality. J Heart Valve Dis 1996;5:26-30. [PubMed]
- Ratnatunga CP, Edwards MD, Dore CJ, et al. Tricuspid valve replacement: UK heart valve registry mid-term results comparing mechanical and biological prosthesis. Ann Thorac Surg 1998;66:1940-7. [Crossref] [PubMed]
- Kaplan M, Kut MS, Demirtas MM, et al. Prosthetic replacement of tricuspid valve: bioprosthesis or mechanical? Ann Thorac Surg 2002;73:467-73. [Crossref] [PubMed]





