Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis
Introduction
Without interventional treatment, symptomatic patients with severe aortic valve stenosis have a dismal prognosis with a one-year mortality of 30-50% (1-3). Since the introduction of percutaneous pulmonary valve implantation in 2000 (4) and subsequent aortic valve implantation in 2002 (5), technological advances in transcatheter aortic valve implantation (TAVI) has affirmed its emergence as a potential alternative treatment modality to conventional surgical aortic valve replacement (AVR) in selected patients (6) (Figure 1) .
Although there are cumulative data suggesting superior survival and symptomatic outcomes for inoperable patients who undergo TAVI versus medical palliation (3,7), the comparative results of high surgical risk patients who undergo TAVI versus AVR remains controversial. Despite widespread enthusiasm and an exponential growth in the utilization of this novel technique in Europe and North America, there is a lack of robust clinical evidence comparing TAVI with the current standard of treatment, which remains to be conventional surgical AVR, in patients who are deemed to be operable candidates.
The present systematic review and meta-analysis aims to identify and compare all relevant data on TAVI versus AVR in the current literature. The primary endpoint is all-cause mortality during the periprocedural period, defined as 30-days or during the same hospitalisation (whichever is longer), all-cause mortality at 1-year, and beyond 1-year. Secondary endpoints include a number of outcomes described in the Valve Academic Research Consortium (VARC) standardized endpoint definitions (8). Progressive changes in transvalvular gradients measured by echocardiography were also compared between the two groups at baseline and after treatment.
Methods
Search strategy and selection criteria
Electronic searches were performed using Ovid Medline, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from 1 January, 2000 to 15 July, 2012. To achieve the maximum sensitivity of the search strategy and identify all studies, we combined the terms “transcatheter” or “transapical” or “transfemoral” or “transcutaneous” or “transvascular” or “percutaneous” with “aortic valve” or “aortic valve stenosis” as either key words or MeSH terms. After initial screening based on titles and abstracts, the full text of potentially relevant studies were obtained for further evaluation. The reference lists of all retrieved articles were reviewed for further identification of relevant studies.
Eligible comparative studies for the present systematic review and meta-analysis included those in which data were available for patients with severe aortic stenosis who were treated by TAVI or AVR. All forms of TAVI were included, as were patients who underwent surgical AVR using different valves. For studies that included patients with aortic stenosis who were treated medically as a subset of patients with aortic stenosis, outcomes for patients who underwent TAVI and AVR were extracted when possible. When centers have published duplicate trials with accumulating numbers of patients or increased lengths of follow-up, the most complete reports were included for qualitative appraisal. To maintain the consistency of measured endpoints, the VARC endpoint definitions were used as a guideline to assess short-term outcomes when applicable (8). All publications were limited to human subjects and in English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted due to potential publication bias and possible duplication of results. Studies that included fewer than twenty patients in either treatment group or presented data with less than 30-days follow-up were also excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators (S.A. and P.I.) independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigators (C.C. and T.D.Y.).
Statistical analysis
Meta-analysis was performed by combining the results of reported incidences of the predetermined endpoints. The relative risk (RR) was used as a summary statistic. In the present study, both fixed and random effect models were tested. In a fixed effect model, it was assumed that treatment effect in each study was the same, whereas in a random effect model, it was assumed that there were variations between studies and the calculated ratios thus had more conservative value (9). χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies due to heterogeneity rather than chance. I2 can be calculated as: I2 =100% × (Q-df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom (10). An I2 value of greater than 50% was considered to represent substantial heterogeneity. If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. In the present meta-analysis, the results using the random-effects model were presented to take into account the possible clinical diversity and methodological variation amongst studies. Specific analyses considering confounding factors were not possible because raw data were not available. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.1.2 (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Results
Quantity and quality of trials
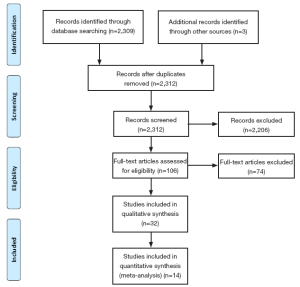
A total of 2,309 references were identified through the five electronic database searches. After exclusion of duplicate or irrelevant references, 106 potentially relevant articles were retrieved for more detailed evaluation. Manual search of the reference lists identified three additional relevant studies. After applying the selection criteria, 32 comparative studies remained for assessment (11-42). A summary of study characteristics is presented in Table 1. Ten studies were excluded due to duplicating patients at different follow-up periods (13,16,17,21,28,30,31,35-37) and eight studies were excluded because the primary endpoint data was not available (15,18,23,24,32,33,40,42). The study selection process is presented in Figure 2 according to the PRISMA statement (43).
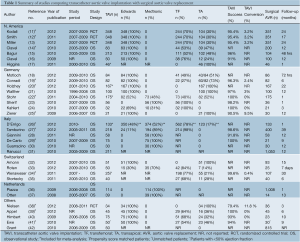
Full table
Of the 14 studies included in the present meta-analysis, three studies reported outcomes from two randomized controlled trials at different time intervals, and 11 were from observational studies. In these 14 studies, 3,465 patients with severe aortic stenosis were compared, including 1,688 patients who underwent TAVI and 1,777 patients who underwent AVR. Follow-up period ranged widely from two days to two years. A summary of baseline patient characteristics, risk factors and risk stratification scores in each study, including the Society of Thoracic Surgeons (STS) score and logistic Euroscore, is presented in Table 2.
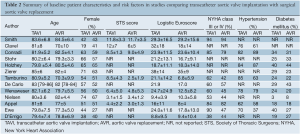
Full table
Procedural technique
Two commercial TAVI devices were used in all studies, including the self-expandable CoreValve porcine pericardial device (Medtronic, Inc., Minneapolis, Minnesota) and the balloon-expandable Edwards SAPIEN bovine pericardial device (Edwards Life Sciences, Irvine, California). The Edwards SAPIEN valve can either be delivered percutaneously or via a transapical route. Direct comparisons between the two approaches was not feasible as a ‘transfemoral-first’ patient selection process was implemented in a number of institutions, whereby the transapical approach was reserved for patients who were more likely to have severe systemic vascular disease and other comorbidities (11,12,19). A summary of commercial devices used and the vascular approach of TAVI deployment is included in Table 1.
Assessment of mortality
All-cause mortality was not significantly different between the TAVI and AVR treatment groups during the periprocedural period [7.5% vs. 6.9%; RR, 1.13; 95% confidence interval (CI), 0.88-1.46; P=0.33; I2=3%], as seen in Figure 3. Similarly, no significant differences were identified at 12 months (18.9% vs. 16.0%; RR, 1.06; 95% CI, 0.87-1.30; P=0.55; I2=3%) or beyond 12 months (28.8% vs. 30.1%; RR, 1.02; 95% CI, 0.85-1.23; P=0.82; I2=0%).
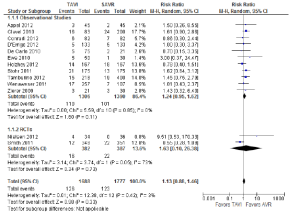
Cardiovascular related mortality was also not significantly different between TAVI and AVR during the periprocedural period (3.7% vs. 3.6%; RR, 0.89; 95% CI, 0.54-1.47; P=0.65; I2=0%), 12 months (12.8% vs. 11.3%; RR, 1.16; 95% CI, 0.83-1.61; P=0.39; I2=0%), or beyond 12 months (17.7% vs. 15.5%; RR, 1.19; 95% CI, 0.90-1.58; P=0.22; I2=0%).
Assessment of stroke
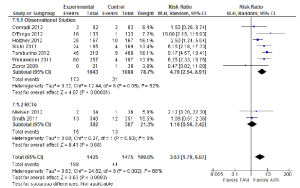
The incidence of stroke was not significantly different between TAVI and AVR during the periprocedural period (2.6% vs. 2.3%; RR, 1.16; 95% CI, 0.72-1.87; P=0.54; I2=0%), at 12 months (4.5% vs. 3.4%; RR, 1.27; 95% CI, 0.68-2.37; P=0.46; I2=29%) or beyond 12 months (5.8% vs. 4.1%; RR, 1.44; 95% CI, 0.82-2.53; P=0.21; I2=5%). The periprocedural stroke outcomes are presented in Figure 4.
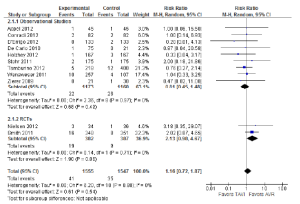
When a combination of stroke or transient ischaemic attacks (TIA) was assessed, patients who underwent TAVI did not have a significantly different incidence compared to patients who underwent AVR in the periprocedural period (4.6% vs. 3.9%; RR, 1.08; 95% CI, 0.43-2.72; P=0.87; I2 =64%). However, subgroup analysis of the two RCTs identified a significantly higher incidence of stroke or TIA for the TAVI cohort (5.8% vs. 2.3%; RR, 2.48; 95% CI, 1.16-5.31; P=0.02; I2 =0%), a finding that was inconsistent with data reported in observational studies (3.5% vs. 6.2%; RR, 0.55; 95% CI, 0.27-1.11; P=0.10; I2 =0%).
Other perioperative outcomes
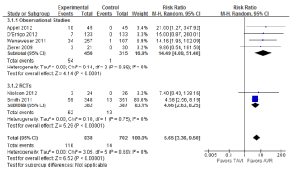
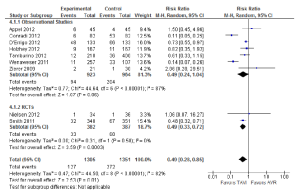
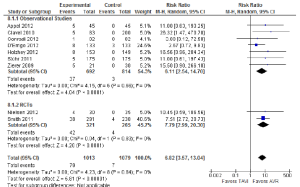
A number of perioperative outcomes were measured according to the VARC endpoint definitions (8). The incidence of vascular complications was significantly higher in patients who underwent TAVI compared to AVR (13.8% vs. 2.0%; RR, 5.65; 95% CI, 3.36-9.50; P<0.00001; I2=0%), as seen in Figure 5. Conversely, major bleeding occurred less frequently after TAVI compared to AVR (9.7% vs. 20.1%; RR, 0.49; 95% CI, 0.28-0.85; P=0.01; I2=82%), as seen in Figure 6. There were no significant differences in the incidences of myocardial infarction (0.5% vs. 0.5%; RR, 0.89; 95% CI, 0.31-2.59; P=0.84; I2=0%) or acute renal failure (6.5% vs. 5.3%; RR, 1.18; 95% CI, 0.57-2.44; P=0.66; I2=68%). Patients were found to require permanent pacemaker insertion significantly more often after TAVI compared to AVR (13.2% vs. 3.0%; RR, 3.53; 95% CI, 1.79-6.97; P=0.0003; I2=68%), as seen in Figure 7.
Echocardiography outcomes
The incidence of postoperative moderate or severe aortic regurgitation, which included both paravalvular and transvalvular regurgitation, was significantly higher after TAVI than AVR (7.8% vs. 0.6%; RR, 6.82; 95% CI, 3.57-13.04; P<0.00001; I2 =0%), as seen in Figure 8. Six studies provided data on transvalvular mean pressure gradient values at baseline and after TAVI or AVR during the periprocedural period and/or at 12 months (12,14,19,26,27,39). A graphic summary of these mean values are presented in Figures 9A (TAVI) and 9B (AVR), demonstrating considerable improvements in mean pressure gradient values after both procedures during the periprocedural period and beyond.

Discussion
In developed countries, aortic stenosis is most commonly caused by calcification of the aortic valve, secondary to a pathophysiological process similar to atherosclerosis (44). With an aging population, the prevalence of symptomatic patients with severe aortic stenosis and their individual surgical risk for aortic valve replacement are likely to increase in the foreseeable future. Since the first human percutaneous aortic valve implantation was performed less than a decade ago, there has been a heightened interest in the application of this technique by both cardiologists and cardiothoracic surgeons (6). In recent years, TAVI has emerged as a viable alternative treatment option for patients considered inoperable by conventional AVR (3). This was reflected by the Food and Drug Administration approval of the Edwards SAPIEN device in November 2011. The key question in the current medical setting is whether the procedure will benefit patients with severe aortic stenosis who are deemed operable by conventional aortic valve replacement, but are considered to have a high surgical risk. To answer of this question, multiple factors need to be considered. Firstly, the definition of ‘high operative risk’ needs to be established, and the risk assessment models for patients undergoing AVR need to be refined. Secondly, the safety profiles of TAVI in this group of patients should be critically assessed. Thirdly, robust clinical endpoints need to be measured to identify any potential benefit of TAVI in comparison to surgical AVR. With these questions in mind, the present study is the first systematic review and meta-analysis to compare TAVI with surgical AVR in patients with severe aortic stenosis.
The present systematic review demonstrated a few deficiencies in the current literature, which need to be addressed in the future trials. Firstly, the definitions of ‘high surgical risk’ and the risk score models utilized were inconsistent amongst the identified studies. A number of parameters have been used to define this subgroup without robust supporting evidence-based data. The patient selection criteria for TAVI varied between institutions, and included age >75 (19,20,25,29,38) or >80 (34), aortic valve area of <1.0 cm2 (14,32,34) or <0.8 cm2 (11,12,35), mean pressure gradient ≥40 mmHg (22,34), logistic Euroscore >20% (19,22,25) or >15% (34), additive Euroscore ≥9 (20,21), or STS score >15% (29) or >10% (11,12). In some institutions, patients who were deemed ‘too high risk’ were also excluded, including those who had an ejection fraction of <20% (11,12) or <15% (29). Furthermore, some centers modified their patient selection criteria for TAVI during their study period due to unexpected outcomes (38). The patient selection process for surgical AVR was not described in detail in the majority of studies. Even though there is widespread dissatisfaction with historical surgical risk stratification scores such as the Euroscore and STS score, a novel clinical risk score for TAVI candidates remains elusive (14,19,25,26). The absence of an accurate and widely accepted preprocedural risk assessment system presents a significant challenge to establish stringent patient selection criteria and to allow meaningful outcome comparisons between institutions. The heterogeneous and subjective definitions of ‘high surgical risk’ need to be acknowledged and a concerted effort by the TAVI community is required to establish a clearer classification of this subgroup of patients. To facilitate this process, a cross-sectional survey is currently underway to identify the accepted definition of ‘high surgical risk’ in patients with severe aortic stenosis (45).
The present meta-analysis did not identify any significant differences in the incidence of all-cause mortality and stroke/TIA between the two treatment modalities. A number of limitations in the current literature may account for these findings and the results must be interpreted with caution. Firstly, some studies did not utilize an intention-to-treat analysis, and a number of institutions excluded patients from statistical analysis in the TAVI group after poor outcomes during the periprocedural period, including patients who had unsuccessful implantations or perioperative deaths (14,21,27,28). Secondly, crossovers from TAVI to AVR were not explicitly reported in all studies, and patients who underwent surgical AVR after an unsuccessful TAVI were not analyzed in some studies (20,34). Such exclusions may have a significant impact on the overall outcomes and skew the results in favor of TAVI.
Furthermore, the reporting of periprocedural adverse outcomes, especially stroke, has been variable in definition and surveillance. The PARTNER trial identified a significantly higher incidence of stroke or TIA at 30-days,1-year and 2-years for patients who underwent TAVI compared to AVR. However, Kodali and colleagues acknowledged that stroke assessments were limited in their study, since neurologic assessments were not mandated (11). Even so, the incidences of periprocedural stroke and TIA in the TAVI cohort were relatively high in the two prospective, randomized controlled trials compared to other observational studies. Authors of the PARTNER trial emphasized the difficulty in assessing stroke outcomes after TAVI in observational studies due to a paucity of independent adjudication in most self-reporting databases that may lack objective definitions and universal auditing (11). Concerns regarding cerebral embolic events in patients who undergo TAVI have been highlighted in a study involving thirty patients who underwent pre-TAVI and post-TAVI magnetic resonance imaging, which demonstrated new embolic lesions in 73% of patients (46).
Other important findings from the present meta-analysis revealed that major vascular complications occurred in one in every seven patients who underwent TAVI, which was seven times more frequent than surgical AVR. There was also a similar proportion of patients who required the insertion of a permanent pacemaker after TAVI, which was also significantly more likely than patients who underwent AVR. Major bleeding was reported in approximately one in every five patients who underwent surgical AVR, twice as common as those who were treated by TAVI. Both treatment modalities were shown to significantly decrease the aortic valve mean pressure gradient during the periprocedural period and beyond. However, patients who underwent TAVI were much more likely to have moderate or severe aortic regurgitation, including paravalvular regurgitation, which has been shown to be associated with reduced long-term survival (11).
To date, two randomized studies have compared TAVI with AVR. Cohort A of the Placement of Aortic Transcatheter Valves (PARTNER) trial involved 25 centers and randomized 699 high risk patients with severe aortic stenosis to either TAVI (n=348) or AVR (n=351). Results at 12 months and two years did not identify any significant differences in all-cause mortality or stroke. However, patients who underwent TAVI were more likely to have stroke or TIA than patients who underwent AVR (11,12). Ethical, scientific and industry-related challenges to the PARTNER trial have recently been highlighted by an independent analysis, citing publication bias, lack of data transparency, unbalanced patient characteristics and incompletely declared conflicts of interest (47). Despite these criticisms, the PARTNER trial represents the largest and the only completed randomized study to date. The more recent STACCATO trial was conducted in two Danish centers after initial encouraging results from early institutional experience (38,48). Compared to the PARTNER trial, patients initially recruited in this study had a lower surgical risk and all patients underwent the transapical TAVI approach rather than the transfemoral approach. Although 200 patients were planned for inclusion in the study, the STACCATO trial was prematurely terminated upon advice of the Data Safety Monitoring Board due to unexpectedly poor outcomes in the TAVI cohort (n=34), compared to the SAVR cohort (n=36) (38). Authors of this trial concluded that current indications for TAVI should remain restricted to surgically inoperable patients only.
Apart from the two randomized trials, the remaining comparative studies included in the present meta-analysis were eleven observational studies. Of these, seven studies were retrospective institutional analyses that were inherently associated with potential confounding factors (14,19,20,22,25,39,41). In the remaining four prospective registries, major flaws included a significant loss to follow-up (26), exclusion of patients who had an unsuccessful TAVI procedure (27), inclusion of patients who underwent surgical AVR with concomitant coronary artery bypass graft or mitral valve surgery (29), and exclusion of patients who had crossover treatment (34). In addition, it should be emphasized that the follow-up periods of all studies were relatively short, with only two studies providing detailed outcome data beyond 12 months (11,34). The comparison of long-term efficacy of TAVI versus AVR remains largely unknown and late-onset adverse outcomes have not yet been systemically evaluated.
Heterogeneity was identified in a number of perioperative outcomes, and may partially be due to varying definitions of adverse outcomes. For example, major bleeding included a wide spectrum of inclusion criteria in the PARTNER trial, ranging from fatal bleeding to bleeding that required a transfusion of more than 3 units of blood within 24 hours. Differences in reporting also ranged between studies, including ‘life-threatening’ bleeding (27), requiring re-operation (17), or requiring more than four units of packed cells (34). Similarly, acute renal failure was often defined as requiring dialysis (12,20,22,25,38,39) but stage 3 renal failure in others (27,34). Consideration should also be given to differences in TAVI techniques and patient baseline characteristics.
Of note, at least half of the studies assessed in this meta-analysis have declared a conflict of interest due to affiliation with device companies (11,12,14,20,34,38,41). The largest randomized controlled trial to date was funded by Edwards Lifesciences, which was responsible for institution and patient selection as well as management of clinical data and site monitoring (11,12). This inherent potential conflict of interest may have contributed to conditions that were conducive to the relatively successful outcomes of patients who underwent TAVI compared to other large registries (49-51). Whilst recognizing the significant costs and logistic challenges associated with conducting a large study on a novel procedure, and acknowledging important industry contribution in this endeavor, there should be a conscious effort by cardiac physicians and surgeons in performing a large, well-designed randomized-controlled study without financial support from the medical industry to minimize potential bias to compare TAVI versus AVR.
In conclusion, the present systematic review identified two randomized controlled trials and 11 observational reports comparing TAVI with AVR in patients with severe aortic stenosis. Meta-analysis of selected studies identified no significant differences in mortality and stroke between the two treatment groups. However, vascular complications, permanent pacemaker insertion and significant aortic regurgitation were relatively common after TAVI, and significantly more frequent than after conventional AVR. Conversely, major bleeding was more likely to occur after surgical AVR than TAVI. Future registries and trials should adhere to the VARC endpoint definitions (8). Furthermore, outcomes should be reported by an intention-to-treat analysis, and patients with unsuccessful implantations or adverse outcomes should not be excluded from post-hoc analysis. Important complications such as stroke, which is not only a debilitating adverse outcome but also a significant predictor of mortality, should be mandatory in prospective TAVI registries (52). Ultimately, longer follow-up data must be presented before any definitive conclusions can be established for this potentially revolutionary technique. Currently, the use of TAVI for eligible surgical candidates should be considered within the boundaries of clinical trials with special arrangements for clinical governance, consent, audit and research.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ben-Dor I, Pichard AD, Gonzalez MA, et al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation 2010;122:S37-42.
- Turina J, Hess O, Sepulcri F, et al. Spontaneous course of aortic valve disease. Eur Heart J 1987;8:471-83.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000;356:1403-5.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8.
- Cribier A. Development of transcatheter aortic valve implantation (TAVI): a 20-year odyssey. Arch Cardiovasc Dis 2012;105:146-52.
- Figulla L, Neumann A, Figulla HR, et al. Transcatheter aortic valve implantation: evidence on safety and efficacy compared with medical therapy. A systematic review of current literature. Clin Res Cardiol 2011;100:265-76.
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205-17.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98.
- Miller DC, Blackstone EH, Mack MJ, et al. Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg 2012;143:832-43.
- Clavel MA, Webb JG, Rodés-Cabau J, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010;122:1928-36.
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74.
- Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009;53:1883-91.
- Higgins J, Ye J, Humphries KH, et al. Early clinical outcomes after transapical aortic valve implantation: a propensity-matched comparison with conventional aortic valve replacement. J Thorac Cardiovasc Surg 2011;142:e47-52.
- Motloch LJ, Reda S, Rottlaender D, et al. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg 2012;93:124-31.
- Conradi L, Seiffert M, Treede H, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a propensity score analysis in patients at high surgical risk. J Thorac Cardiovasc Surg 2012;143:64-71.
- Holzhey DM, Shi W, Rastan A, et al. Transapical versus conventional aortic valve replacement--a propensity-matched comparison. Heart Surg Forum 2012;15:E4-8.
- Walther T, Schuler G, Borger MA, et al. Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J 2010;31:1398-403.
- Stöhr R, Dohmen G, Herpertz R, et al. Thirty-day outcome after transcatheter aortic valve implantation compared with surgical valve replacement in patients with high-risk aortic stenosis: a matched comparison. Coron Artery Dis 2011;22:595-600.
- Sherif MA, Abdel-Wahab M, Awad O, et al. Early hemodynamic and neurohormonal response after transcatheter aortic valve implantation. Am Heart J 2010;160:862-9.
- Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010;121:870-8.
- Zierer A, Wimmer-Greinecker G, Martens S, et al. Is transapical aortic valve implantation really less invasive than minimally invasive aortic valve replacement? J Thorac Cardiovasc Surg 2009;138:1067-72.
- D’Errigo P, Barbanti M, Ranucci M, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis: Results from an intermediate risk propensity-matched population of the Italian OBSERVANT study. Int J Cardiol 2012. [Epub ahead of print].
- Tamburino C, Barbanti M, Capodanno D, et al. Comparison of complications and outcomes to one year of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis. Am J Cardiol 2012;109:1487-93.
- Giannini C, Petronio AS, Nardi C, et al. Left ventricular reverse remodeling in percutaneous and surgical aortic bioprostheses: an echocardiographic study. J Am Soc Echocardiogr 2011;24:28-36.
- De Carlo M, Giannini C, Ettori F, et al. Impact of treatment choice on the outcome of patients proposed for transcatheter aortic valve implantation. EuroIntervention 2010;6:568-74.
- Guarracino F, Talini E, Landoni G, et al. Effect of aortic valve surgery on left ventricular diastole assessed by echocardiography and neuroendocrine response: percutaneous versus surgical approach. J Cardiothorac Vasc Anesth 2010;24:25-9.
- Ranucci M, Guarracino F, Castelvecchio S, et al. Surgical and transcatheter aortic valve procedures. The limits of risk scores. Interact Cardiovasc Thorac Surg 2010;11:138-41.
- Amonn K, Stortecky S, Brinks H, et al. Quality of life in high-risk patients: comparison of transcatheter aortic valve implantation with surgical aortic valve replacement. Eur J Cardiothorac Surg 2012. [Epub ahead of print].
- Roten L, Stortecky S, Scarcia F, et al. Atrioventricular conduction after transcatheter aortic valve implantation and surgical aortic valve replacement. J Cardiovasc Electrophysiol 2012;23:1115-22.
- Wenaweser P, Pilgrim T, Kadner A, et al. Clinical outcomes of patients with severe aortic stenosis at increased surgical risk according to treatment modality. J Am Coll Cardiol 2011;58:2151-62.
- Stortecky S, Brinks H, Wenaweser P, et al. Transcatheter aortic valve implantation or surgical aortic valve replacement as redo procedure after prior coronary artery bypass grafting. Ann Thorac Surg 2011;92:1324-30; discussion 1230-1.
- Piazza N, van Gameren M, Jüni P, et al. A comparison of patient characteristics and 30-day mortality outcomes after transcatheter aortic valve implantation and surgical aortic valve replacement for the treatment of aortic stenosis: a two-centre study. EuroIntervention 2009;5:580-8.
- Otten AM, van Domburg RT, van Gameren M, et al. Population characteristics, treatment assignment and survival of patients with aortic stenosis referred for percutaneous valve replacement. EuroIntervention 2008;4:250-5.
- Nielsen HH, Klaaborg KE, Nissen H, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012;8:383-9.
- Appel CF, Hultkvist H, Nylander E, et al. Transcatheter versus surgical treatment for aortic stenosis: patient selection and early outcome. Scand Cardiovasc J 2012;46:301-7.
- Himbert D, Descoutures F, Al-Attar N, et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol 2009;54:303-11.
- Ewe SH, Ajmone Marsan N, Pepi M, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J 2010;160:1113-20.
- Grant SW, Devbhandari MP, Grayson AD, et al. What is the impact of providing a transcatheter aortic valve implantation service on conventional aortic valve surgical activity: patient risk factors and outcomes in the first 2 years. Heart 2010;96:1633-7.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956-66.
- Cao C, Yan TD. Cross-sectional Survey on TAVI Study. Ann Cardiothorac Surg 2012. [Epub ahead of print]
- Ghanem A, Müller A, Nähle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 2010;55:1427-32.
- Van Brabandt H, Neyt M, Hulstaert F. Transcatheter aortic valve implantation (TAVI): risky and costly. BMJ 2012;345:e4710.
- Nielsen HH, Thuesen L, Egeblad H, et al. Single center experience with transcatheter aortic valve implantation using the Edwards SAPIEN™ Valve. Scand Cardiovasc J 2011;45:261-6.
- Gotzmann M, Pljakic A, Bojara W, et al. Transcatheter aortic valve implantation in patients with severe symptomatic aortic valve stenosis-predictors of mortality and poor treatment response. Am Heart J 2011;162:238-245.
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J 2011;32:198-204.
- Redberg RF, Dhruva SS. Transcatheter aortic-valve replacement. N Engl J Med 2011;365:958-9; author reply 959.
- Eggebrecht H, Schmermund A, Voigtländer T, et al. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention 2012;8:129-38.