Robotic mitral valve surgery: current limitations and future directions
Introduction
The primary aim of robotic mitral valve surgery is simple: to replicate a high quality, sternotomy-based mitral valve operation with a less invasive approach. With robotic mitral valve surgery, the integrity of the operation—its safety and effectiveness—remains intact. When compared to sternotomy-based surgery, robotically-assisted mitral valve operations have the clinical advantages of reduced blood loss, lower risk of incisional infection and shorter hospital length of stay (1-3). From the patient perspective, the advantages include quicker return to full function and a superior cosmetic result.
Given that a number of studies confirm these features of robotic surgery, why hasn’t the robotic approach become standard? The answer to this question lies in the limitations, both perceived and real, of robotically assisted surgery. Surgeons express concerns over patient safety, mitral valve repair rates, procedural complexity and the learning curve. In addition, certain patient characteristics, including mitral annular calcification, aortic regurgitation and aortoiliac atherosclerosis, are relative contraindications to robotically-assisted mitral valve surgery. Although many of these limitations are grounded more in perception than in reality, each requires careful consideration, and some represent opportunities for innovation and improvement.
Perceived limitations
Safety: is robotic mitral valve surgery safe?
Of course, the answer is ‘yes’. However, as with all medical procedures, the safety of robotic surgery is contingent upon appropriate patient selection and excellent procedural technique.
Concerns over the safety of robotic mitral valve surgery stem in large part from a study of minimally invasive mitral valve surgery based on the Society of Thoracic Surgeons (STS) database (4). That report documented a twofold increase in the risk of stroke with less invasive mitral valve surgery. Often used as an indictment of robotic and minimally invasive mitral valve surgery, this study had several features that render it largely irrelevant to the current discussion of robotic mitral valve surgery. Firstly, it contained little information that pertains specifically to the use of the surgical robot. Rather, it included a wide variety of patients undergoing numerous different procedures via varied incisions and techniques. Because the study used femoral cannulation as a surrogate for minimally invasive mitral valve surgery, it is likely that it included patients in whom atherosclerosis precluded safe aortic cannulation (i.e., porcelain aorta). Such patients would be expected to have less favorable outcomes. Secondly, the fibrillating heart technique was identified as a risk factor for stroke. This approach should be employed only rarely in the robotic setting.
A large number of single center series have confirmed the safety of robotic mitral valve surgery in experienced centers (1,2,5-8). Hospital mortality is generally less than 1% and the stroke rate is similarly low. In addition, a recent analysis from the STS database failed to identify particular safety concerns with robotic mitral valve surgery (3).
In our opinion, safety begins with patient selection. In the preoperative evaluation, all potential robotic candidates undergo coronary angiography, transthoracic echocardiography, and computed tomography (CT) scanning of the chest, abdomen and pelvis. These three tests enable determination of a patient’s candidacy for robotically-assisted mitral valve surgery. Using these studies to guide patient selection optimizes safety (Table 1).
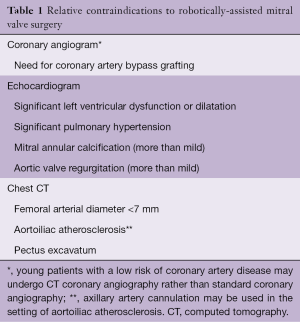
Full table
Repair rate: does robotic mitral valve surgery compromise mitral valve repair?
The answer is ‘no’. The robot is nothing more than a surgical instrument. The ability to repair the valve rests with the surgeon. Virtually all valves that can be repaired with an open approach can also be repaired using the surgical robot, with the possible exception of mitral annular calcification, as discussed below. A surgeon must be an expert at mitral repair before adopting the robotic approach. Surgeons should develop their repair skills via sternotomy-based approach before attempting to incorporate the surgical robot into their practice.
Real limitations
Complexity: does robotic mitral valve surgery introduce complexity?
The answer to this question is ‘yes’. Additional areas of complexity introduced by robotic mitral valve surgery include port placement, management of peripheral cardiopulmonary bypass and myocardial protection.
Optimal port placement is critically important. Improper placement can lead to conflicts between the three robotic arms and the camera, limiting motion and the ability to work on the mitral valve. As a general rule, the working port is created in the fourth interspace and the other ports are positioned based upon this port and an appreciation of the patient’s individual anatomy. No fixed rules for port location ensure an absence of conflicts. With increasing experience, the surgeon develops a ‘feel’ for port placement that facilitates the operation. Early experience suggests that the latest iteration of the surgical robot reduces instrument conflicts.
Robotic mitral valve surgery entails peripheral cannulation for cardiopulmonary bypass. Although arterial perfusion via the femoral artery is the convention, the surgeon may choose to use the axillary artery in the setting of small femoral vessels (<7 mm) or aortoiliac atherosclerosis. However, the presence of small femoral vessels generally implies a small axillary artery as well. The necessity for peripheral perfusion introduces the possibility of femoral complications (vessel damage, hematoma, lymphocele, seroma, nerve damage). Prolonged femoral perfusion may also create limb ischemia. To prevent this, many surgeons place a separate small cannula for distal limb perfusion and/or monitor limb perfusion during the operation.
In most cases, excellent venous drainage can be obtained by advancing a cannula from the femoral vein into the superior vena cava. By employing a cannula with multiple holes, this placement enables drainage of the superior vena cava, right atrium and inferior vena cava. In order to ensure optimal drainage in all patients, we also place a second venous cannula via the right internal jugular vein. The anesthesia team performs a ‘double stick’ of the right internal jugular vein, and uses the Seldinger technique to exchange this for a venous cannula.
In robotic mitral valve surgery, the surgeon can choose between all available options for myocardial protection. An antegrade cardioplegia catheter placed in the proximal ascending aorta enables excellent myocardial protection with standard cardioplegia solutions. In addition, many surgeons elect to supplement this with a retrograde cardioplegia catheter placed with echocardiographic or fluoroscopic guidance via the internal jugular vein. In some cases, coronary sinus access can be challenging. Use of a ‘single shot’ cardioplegia solution (e.g., Del Nido cardioplegia) simplifies myocardial protection by eliminating the need to halt the operation to give additional doses of cardioplegia. Table 2 outlines the simplest approach to cardiopulmonary bypass and myocardial protection.
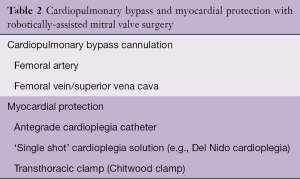
Full table
Aortic regurgitation: does aortic regurgitation create safety issues for the patient undergoing robotically-assisted mitral valve surgery?
The answer to this question depends upon the degree of aortic regurgitation. Aortic regurgitation can create problems with myocardial protection. If aortic regurgitation is more than mild, antegrade cardioplegia is generally ineffective. In addition, placement of a Chitwood clamp or an intra-aortic balloon occlusion occasionally distorts the aortic valve, temporarily increasing the degree of aortic regurgitation. Therefore, in the patient with any degree of aortic regurgitation, it is prudent to place a coronary sinus catheter via the right internal jugular vein to provide a secure, alternate route for cardioplegia delivery.
When the aortic cross-clamp is removed, aortic regurgitation may cause ventricular distension and render defibrillation problematic; leaving a vent across the repaired mitral valve until the heart is beating helps to alleviate this problem.
Given these two issues—myocardial protection and ventricular distension—we consider more than mild aortic regurgitation to be a contraindication to robotically assisted mitral valve surgery.
Mitral annular calcification: can the surgeon repair a calcified mitral valve using the robot?
The robot does not come equipped with a tool for removal of calcium. Therefore, if preoperative studies indicate that successful valve repair will require debridement of mitral annular calcium, a sternotomy approach is most appropriate. This is particularly important in the setting of severe mitral annular calcification. That said, in many patients with focal calcification, repair can be achieved without calcium debridement; in such cases, robotically-assisted mitral valve repair is feasible.
Expense: does robotically-assisted mitral valve surgery increase cost?
In general, the answer is ‘yes’. At the Cleveland Clinic, we found that application of the surgical robot increased hospital costs marginally while decreasing patient length of stay and time to return to work (9). Focusing on cost reduction, Suri et al. demonstrated that with a concerted effort, the cost of robotically-assisted surgery can be reduced to a level that matches the cost of standard, sternotomy-based surgery (10). At the inception of a robotic program, operative time and cost will exceed those associated with sternotomy. However, with experience and a focus on efficiency, operative time and cost can approximate those of the standard approach.
Conclusions
When operating upon the patient with degenerative mitral valve disease, the surgeon’s objective is to conduct a safe operation that concludes with an excellent mitral valve repair. The chest wall approach—sternotomy, partial sternotomy, right thoracotomy or robotically-assisted procedure—is of secondary importance. In experienced hands, application of the surgical robot satisfies the primary objectives of safety and effectiveness. Limitations of robotically-assisted surgery mandate careful patient selection. This means choosing a conventional approach for the patient with aortic regurgitation that is more than mild and for the patient with pronounced mitral annular calcification. As with all surgical procedures, good surgical judgment illuminates the path to an excellent outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Gillinov discloses the following relationships—Honoraria for speaking (Intuitive Surgical, Medtronic, Edwards Lifesciences, AtriCure, ClearFlow); Consultant (On-X, Abbott); Equity interest (ClearFlow); Research support (St. Jude Medical). The other authors have no conflicts of interest to declare.
References
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Jalbert J, et al. A population-based analysis of robotic-assisted mitral valve repair. Ann Thorac Surg 2015;99:1546-53. [Crossref] [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 1410.e1; discussion 1408-10.
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [Crossref] [PubMed]
- Murphy DA, Moss E, Binongo J, et al. The Expanding Role of Endoscopic Robotics in Mitral Valve Surgery: 1,257 Consecutive Procedures. Ann Thorac Surg 2015;100:1675-81; discussion 1681-2.
- Ramzy D, Trento A, Cheng W, et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228-35. [Crossref] [PubMed]
- Suri RM, Taggarse A, Burkhart HM, et al. Robotic Mitral Valve Repair for Simple and Complex Degenerative Disease: Midterm Clinical and Echocardiographic Quality Outcomes. Circulation 2015;132:1961-8. [Crossref] [PubMed]
- Mihaljevic T, Koprivanac M, Kelava M, et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg 2014;149:679-86. [Crossref] [PubMed]
- Suri RM, Thompson JE, Burkhart HM, et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin Proc 2013;88:1075-84. [Crossref] [PubMed]





