Catheter-based or surgical repair of the highest risk secondary mitral regurgitation patients
Introduction
Secondary or functional mitral regurgitation (MR) is caused by a primary disease of the left ventricular (LV) myocardium which leads to LV dilatation due to ischemic or non-ischemic dilated cardiomyopathy. LV dilatation leads to apical and lateral papillary muscle displacement, resulting in leaflet tethering, as well as dilatation and flattening of the mitral annulus. These pathological changes of secondary MR induce leaflet coaptation failure and decreased valvular closing forces despite a structurally normal mitral valve (MV).
Whether surgical mitral repair is beneficial for patients with secondary MR or only corrects an echocardiographic diagnosis without treating the underlying disease remains controversial. Recently, less-invasive, catheter-based mitral repair, specifically with the MitraClip device (Abbott Vascular, Inc., Menlo Park, CA), has been widely implemented in high-risk patients, adding to therapeutic decision-making complexity even further. The purpose of this article is to review the existing methods for MV intervention and to discuss which therapeutic approach might be most advantageous in high-risk secondary MR patients.
Surgery in secondary MR
In the most recent European guidelines [2012] for the treatment of valvular heart disease, the only class I (Level of Evidence: C) recommendation regarding surgery for secondary MR is assigned to patients with severe secondary MR undergoing coronary artery bypass graft (CABG) with a left ventricular ejection fraction (LVEF) of >30% (1). Surgery for isolated severe secondary MR is a class IIb (LOE C) recommendation for patients who remain symptomatic despite optimal medical therapy and who are at low surgical risk with LVEF >30%. In the 2014 American Heart Association/American College of Cardiology (AHA/ACC) Valvular Heart Disease Guidelines, similar recommendations are given (2). Surgical MV operation is recommended for patients with severe (COR IIa, LOE C) or moderate (COR IIb, LOE C) secondary MR who are undergoing CABG as well as severely symptomatic patients (COR IIb, LOE B). In contrast to the European guidelines, preserved or impaired LVEF should not influence decision-making according to the AHA/ACC guidelines.
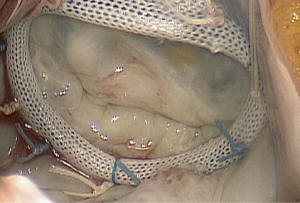
Following these guidelines, surgery is variably performed for secondary MR, although it is still unknown whether correcting MR has any significant positive impact on the underlying LV disease, long-term survival or quality of life (3-6). The most commonly performed surgical technique to restore MV competence is MV annuloplasty (Figure 1). By ‘undersizing’ the annuloplasty ring and overcorrecting annular dilatation, which is greatest in the septolateral (or antero-posterior) diameter, the effects of leaflet tethering are reduced, and leaflet coaptation is restored. Even though initial results show good outcomes in terms of longevity of secondary MR abolishment with mild or less MR, moderate or greater MR recurs in 15-33% of patients at 6-12 months and in up to 70% at five years after MV annuloplasty (6). According to several reports, MR recurrence is more frequent when using flexible rings or partial bands (7-9). Further risk factors for recurrence include a greater degree of LV dilatation, greater degrees of leaflet tethering (posterior lateral angle >45˚), higher degree of preoperative MR, basal aneurysm or dyskinesis, and coaptation height ≥11 mm (10-12).
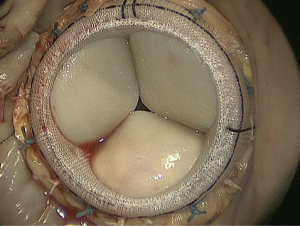
In patients with a high likelihood of MR recurrence, replacement might be superior to repair (13). In MV replacement, the valve-sparing technique should be performed to retain the leaflets and subvalvular apparatus, resulting in preserved LV function through the maintenance of annulo-ventricular continuity (Figure 2). However, there may be a tradeoff between better long-term freedom from recurrent MR after replacement and reduced perioperative morbidity and mortality after repair (14-16). A recently published, randomized study with 251 patients confirmed more durable MR correction with replacement, but showed no difference in clinical outcomes in terms of major adverse events, functional status, LV remodeling as measured by the LV systolic volume index (LVESVI) or quality of life after one year (12).
Because of the lack of conclusive evidence demonstrating benefit, many patients with severe secondary MR are never referred for surgery. Mirabel et al. showed that, despite the poor prognosis of medical therapy, 49% of patients with severe MR do not undergo surgery (17). Of 814 patients with severe secondary MR treated between 2000-2008 at the Cleveland Clinic, only 36% underwent surgery. In addition, 90% of all non-operated patients with severe MR were suffering from secondary MR (18). Nevertheless, though a recent randomized study demonstrated a reduced prevalence of moderate or severe regurgitation at one year following MV repair, there was an increased number of adverse events in the surgical cohort (19).
In summary, MV surgery is recommended for patients with severe secondary MR undergoing cardiac surgery for other reasons, but both the optimal surgical technique (repair versus replacement) and the indication for treatment of isolated MR remain controversial and not clearly defined. Furthermore, patients with moderate secondary MR at the time of CABG should not necessarily undergo MR correction as demonstrated in the most recent NIH CTSN trial. As a result of these controversies, some patients with secondary MR who may otherwise benefit from surgical therapy are currently not being offered surgery. Less invasive techniques with reduced perioperative risk might influence decision making in favor of correction of MR; therefore, transcatheter options to treat patients with MV disease especially at higher risk are being developed.
Transcatheter devices for secondary MR
Transcatheter devices for MR therapy imitate a variety of surgical approaches. Most procedures are modeled after direct or indirect annuloplasty, edge-to-edge repair, chordal replacement or complete MV replacement (20). The MitraClip device, which is modeled after the surgical edge-to-edge repair (Alfieri stitch), is the only device in widespread clinical use, with over 19,000 implantations performed worldwide. In addition, it has received CE Mark approval (for both primary and secondary MR) in Europe and FDA approval (for primary MR, but not secondary MR) in the U.S. Other devices are designed to treat secondary MR by direct mitral remodeling such as the Cardioband device (Valtech Cardio Ltd., Boston, MA) or Mitralign system (Mitralign, Inc., Tewksbury, MA) and are currently undergoing feasibility studies. Another device, Carillon Contour System (Cardiac Dimensions, Kirkland, WA), corrects MR by an indirect annuloplasty technique from placement in the coronary sinus. It has received CE Mark approval for commercial sale in Europe. Newer transcatheter mitral valve replacement (TMVR) may become an acceptable alternative to treat patients at higher-risk with secondary MV disease. By the end of 2014, such devices have been used in only approximately 20 patients worldwide, using three different valves (Fortis, Edwards Lifesciences, Irvine, CA, Figure 3A); Tiara, (Neovasc Inc, Richmond, BC, Canada, Figure 3B); and CardiaQ, (CardiaQ Valve Technologies, Irvine, CA, Figure 3C). At present, the MitraClip procedure is the only true, interventional alternative to conventional surgery with evidence of possible clinical benefit. Therefore, the remainder of this article is focused solely on the MitraClip procedure and its outcomes.
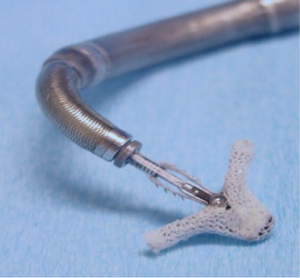
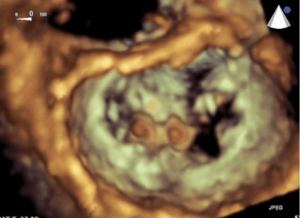
The MitraClip device is a polyester-covered, cobalt-chromium clip (Figure 4). Through transfemoral venous access and trans-septal puncture, the MitraClip is advanced into the left atrium, then opened and positioned directly above the regurgitant jet (Figure 5). Precision placement has been significantly enhanced by the use of 3D and X-plane transesophageal echocardiography (TEE) guidance. After insertion into the LV, it is retracted, and the free edges of the mitral leaflets are grasped. After fixation by the grippers, the clip is closed. MR reduction can be assessed by TEE. With successful MR reduction, the clip is released. There has been a recent trend to implant multiple clips during one procedure to achieve better outcomes, especially in secondary MR. To date, there are no reported cases of mitral stenosis necessitating intervention, even after multiple clip implantations (21,22). The EVEREST II trial, with 279 relatively low-risk MR patients, compared MitraClip and surgical MV repair. Results demonstrated that the MitraClip is safer but not as effective in reducing MR. Although the study showed a superior efficacy endpoint rate using the MitraClip device compared to surgery in the secondary MR cohort, any definite conclusion regarding benefit cannot be made, as only 44 patients in the MitraClip and 22 in the surgery group were treated (23).
Larger registries with higher proportions of secondary MR treated have demonstrated device success and good safety profile in this patient cohort but no clear benefit. The Transcatheter Mitral Valve Interventions (TRAMI) Registry, with 1,064 Patients (71% suffering from secondary MR), showed that the procedure can be performed with a high success rate (95% device success) with no procedural deaths in a high-risk patient cohort (median STS mortality score 10, and 69% of the patients with LVEF <50%) (24). The European Sentinel Pilot Registry, with a similar rate of secondary MR patients (71% of 628), confirmed these results via a high procedural success (95.4%) and low mortality rate (in-hospital mortality 2.9%) (25). Although the re-hospitalization for heart failure was more common in the secondary MR group compared to primary MR (25.8% vs. 12.0%, P=0.009), recurrence of severe MR was only present in 6% of these patients at one year (25).
Despite the fact that MitraClip has CE mark approval and shows good results in secondary MR patients in large European registries, it only has FDA approval for the treatment of high surgical risk patients with primary MR in the U.S. Thus, in the 2014 ACCF/AHA Valve guidelines, MitraClip is recommended with class IIb (LOE b) guidance for severe primary MR in symptomatic patients at prohibitive risk for MV surgery (2). Three large, ongoing randomized trials comparing MitraClip plus medical therapy versus medical therapy alone in secondary MR patients might help clarify the future role of transcatheter devices in secondary MR therapy, and whether reducing MR improves long-term outcomes. The results of these trials (COAPT, RESHAPE-HF and Mitra-Fr) should be available in 2017. The COAPT Trial in the U.S., in which 430 patients with secondary MR are randomized between guideline-directed medical therapy (GDMT) alone compared with GDMT plus MitraClip with a two year composite endpoint has completed approximately half of its intended enrollment as of early 2015.
Conclusions
Secondary MV disease is associated with ischemic or non-ischemic LV dysfunction and thus carries a higher risk for surgical treatment. Contemporary surgical therapies consist of standard annuloplasty procedures, most commonly with an undersized rigid complete ring, or leaflet-sparing valve replacement. There are few studies on the potential long-term benefits of these therapies currently available. Therefore, recent guidelines recommend surgical MV therapy in only a limited number of patients, typically those who are undergoing additional surgical procedures and possess severe MR.
At this moment, the role of surgical therapy in comparison to transcatheter techniques in secondary MR is not well defined. Both options should be considered in select patients depending on severity of symptoms, patient risk, and a center’s experience with these interventions. Also unclear is whether ischemic and non-ischemic dilated cardiomyopathies respond equally to correction of MR. Any trials or registries analyzing or reporting outcomes should stratify these two different etiologies of secondary MR.
MitraClip therapy has evolved as a valid therapeutic option in higher-risk patients who are not judged by the heart team to be appropriate candidates for conventional procedures. The overall benefits of transcatheter edge-to-edge procedures, however, are not well established. Particularly, recurrence of MR has remained an issue as in surgical annuloplasty.
Therefore, further development of effective TMVR devices may be an attractive therapeutic option in the future. Currently available devices have been implanted into only a few patients, with variable results worldwide. In comparison to the dramatic adoption of transcatheter aortic valve implantations over the past decade, development in transcatheter intervention in the mitral position will be slower due to the much more complex structure of the mitral valve and the lack of proven benefit of even surgical correction of secondary MR. Nevertheless, several devices are being developed for improved transcatheter implantations, and the MV will be one of the most interesting targets for therapeutic cardiac device innovations in the coming years.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57-185. [PubMed]
- Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381-7. [PubMed]
- Silberman S, Oren A, Klutstein MW, et al. Does mitral valve intervention have an impact on late survival in ischemic cardiomyopathy? Isr Med Assoc J 2006;8:17-20. [PubMed]
- Calafiore AM, Iacò AL, Gallina S, et al. Surgical treatment of functional mitral regurgitation. Int J Cardiol 2013;166:559-71. [PubMed]
- Milano CA, Daneshmand MA, Rankin JS, et al. Survival prognosis and surgical management of ischemic mitral regurgitation. Ann Thorac Surg 2008;86:735-44. [PubMed]
- McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916-24. [PubMed]
- Hu X, Zhao Q. Systematic evaluation of the flexible and rigid annuloplasty ring after mitral valve repair for mitral regurgitation. Eur J Cardiothorac Surg 2011;40:480-7. [PubMed]
- Kwon MH, Lee LS, Cevasco M, et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg 2013;146:616-22. [PubMed]
- Calafiore AM, Di Mauro M, Gallina S, et al. Mitral valve surgery for chronic ischemic mitral regurgitation. Ann Thorac Surg 2004;77:1989-97. [PubMed]
- Bach DS, Bolling SF. Improvement following correction of secondary mitral regurgitation in end-stage cardiomyopathy with mitral annuloplasty. Am J Cardiol 1996;78:966-9. [PubMed]
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [PubMed]
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-761.e1.
- Lorusso R, Gelsomino S, Vizzardi E, et al. Mitral valve repair or replacement for ischemic mitral regurgitation? The Italian Study on the Treatment of Ischemic Mitral Regurgitation (ISTIMIR). J Thorac Cardiovasc Surg 2013;145:128-39; discussion 137-8. [PubMed]
- De Bonis M, Ferrara D, Taramasso M, et al. Mitral replacement or repair for functional mitral regurgitation in dilated and ischemic cardiomyopathy: is it really the same? Ann Thorac Surg 2012;94:44-51. [PubMed]
- Vassileva CM, Boley T, Markwell S, et al. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur J Cardiothorac Surg 2011;39:295-303. [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [PubMed]
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014;63:185-6. [PubMed]
- Smith PK, Puskas JD, Ascheim DD, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2014;371:2178-88. [PubMed]
- Chiam PT, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. JACC Cardiovasc Interv 2011;4:1-13. [PubMed]
- Paranskaya L, D'Ancona G, Bozdag-Turan I, et al. Mitral valve repair using multiple MitraClips®: a dobutamine stress echocardiography evaluation. EuroIntervention 2013;8:1372-8. [PubMed]
- Paranskaya L, D'Ancona G, Bozdag-Turan I, et al. Percutaneous mitral valve repair with the MitraClip system: perioperative and 1-year follow-up results using standard or multiple clipping strategy. Catheter Cardiovasc Interv 2013;81:1224-31. [PubMed]
- Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013;62:317-28. [PubMed]
- Schillinger W, Hünlich M, Baldus S, et al. Acute outcomes after MitraClip therapy in highly aged patients: results from the German TRAnscatheter Mitral valve Interventions (TRAMI) Registry. EuroIntervention 2013;9:84-90. [PubMed]
- Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875-84. [PubMed]