Ventricular apical access and closure, and re-access devices to facilitate mitral valve interventions
Introduction
To date, transapical (TA) access has proven to be a safe and effective method of performing transcatheter aortic valve replacement (TAVR) for the treatment of patients with severe aortic stenosis. Current transcatheter mitral valve technologies are forthcoming, with the majority being performed via the TA access route. Current first generation devices will utilize >34F sheaths for deployment of the new mitral technology. It is quite feasible that novel apical access may potentially facilitate access and closure of this high-risk patient population.
Although TA access has a <1% access-related complication rate, the ventricle can be quite difficult to manage when adverse consequences do occur (1,2). The TA technique has several advantages over transfemoral access, including: (I) a short distance to the aortic valve which enables precise control over the device facilitating accurate valve deployment; (II) avoidance of delivery device transit across the aortic arch which may reduce the risk of stroke; (III) no restriction on the sheath size of the delivery device. These characteristics make TA access the ideal platform for future transcatheter mitral valve interventions.
Despite the aforementioned advantages, the increased utilization of TA access has been limited due to the need for a small anterolateral thoracotomy and the potential risk of hemorrhage when closing the apex with sutures. These two limitations have sparked the development of apical access and closure devices that could transform TA access into a more reproducible and percutaneous procedure. This article reviews the current status of these devices.
Devices
Apica ASCTM (Apica Cardiovascular Limited, Galway, Ireland; now Thoratec Corporation)
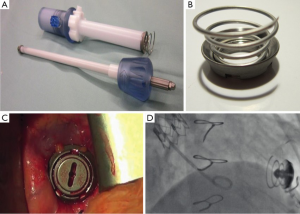
The Apica ASCTM system employs a titanium coil design that provides a working port on the apex of the left ventricle following deployment. There are three parts to the system: an introducer, a left ventricular titanium coil and a closure cap. The delivery system of the TAVR device is placed through the introducer with the titanium coil attached. Once stiff wire apical access is obtained, the TAVR delivery system is loaded on to the wire and advanced with the Apica ASCTM into the apex. The introducer is rotated, inserting the titanium coils into the myocardium. The coils provide myocardial compression and minimize perisheath bleeding during the TAVR procedure. Once the procedure is complete, the TAVR delivery system is removed from the introducer and the closure cap is inserted (Figure 1). The closure cap is removable if the apex needs to be re-accessed for any future interventions. In an initial report of the use of the Apica ASCTM, there was a 100% success rate, with no significant perioperative bleeding or access closure complications (3). This device has obtained CE mark and is commercially available in Europe.
Permaseal (Micro Interventional Devices, Bethlehem, PA)
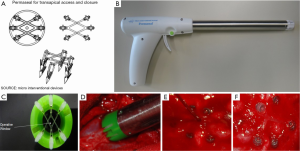
Permaseal is a one-piece device that implants a biocompatible elastomer web for apex closure. After wire access is obtained into the apex, the Permaseal gun-shaped delivery device is guided over the wire and placed against the epicardium. The operator pulls a trigger and six anchors threaded with the elastomer are deployed into the myocardium, creating a series of “V Stays”. This creates compliant and strong webbing that expands to accommodate instruments of different sizes while maintaining tension on the access site. The Permaseal gun is exchanged over the wire for the TAVR delivery system and the procedure is performed. Following valve deployment, all catheters and wires are withdrawn and the elastomer web closes spontaneously to obtain hemostasis (Figure 2). The Permaseal CE mark study evaluating the safety and performance of the device is currently being conducted at six sites within the European Union.
CardiApex (Cardiapex Ltd, Or Akiva, Israel)
The CardiApex system is designed as a percutaneous, sutureless apical closure device. The procedure begins with insertion of a 9F femoral sheath, through which a balloon-tipped catheter equipped with a puncturing needle is advanced retrogradely into the left ventricular cavity. The needle is advanced, puncturing the ventricle from inside to outside the pericardium and an Amplatz Super Stiff wire is advanced outside the pericardium. A 10 mm trocar is percutaneously placed into the sixth intercostal space and the Amplatz wire is snared. Over this wire and through the 10 mm trocar, a sheath is advanced into the left ventricle with a left ventricular balloon inflated around the sheath and an external suction cap placed at the pericardium. This stabilizes the myocardium and allows for the TAVR delivery device to be advanced through this sheath. Once the TAVR is complete, an umbrella-like closure plug is deployed to obtain hemostasis at the apex (Figure 3). The CardiApex feasibility studies have yet to be initiated.
Entourage CardioCloseTM (Entourage Medical Technology, Menlo Park, CA)
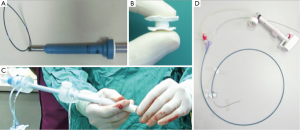
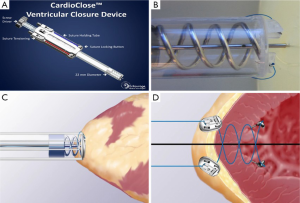
The Entourage CardioCloseTM is a suture-based device that is deployed after wire access is obtained to “pre-close” the apex. This platform employs a double helical needle with two sutures that are inserted into the apex and advanced into the left ventricular cavity. The needles are removed, leaving behind anchors that fix the two sutures to the endocardium. The TAVR delivery sheath is advanced over the wire and TAVR is performed. The delivery sheath is removed and a suture-locking button is placed on each suture. The sutures are tightened and the suture locking buttons are approximated, achieving hemostasis (Figure 4). Feasibility studies using the Entourage CardioClose are underway in Europe.
Summary
TA access will play a vital role in the expansion of transcatheter valve therapy in the treatment of mitral valve pathology. The short distance from the apex to the mitral valve annulus and the ability to accommodate large caliber sheaths make the apex the optimal access point. The continued development of apical access and closure devices will eventually produce a percutaneous device with a small “footprint” which provides a reliable hemostatic seal and does not impact ventricular function.
Acknowledgements
Disclosure: Dr. Thourani has disclosures—Edwards Lifesciences: consultant, research; Apica: IP; Boston Scientific: research, advisory board; Medtronic: research; St. Jude medical: advisory board, research. Dr. Leshnower declares no conflict of interest.
References
- Walther T, Thielmann M, Kempfert J, et al. PREVAIL TRANSAPICAL: multicentre trial of transcatheter aortic valve implantation using the newly designed bioprosthesis (SAPIEN-XT) and delivery system (ASCENDRA-II). Eur J Cardiothorac Surg 2012;42:278-83; discussion 283. [PubMed]
- Walther T, Arsalan M, Kim W, Kempfert J. TAVI: transapical--what else? EuroIntervention 2013;9 Suppl:S19-24. [PubMed]
- Blumenstein J, Kempfert J, Van Linden A, et al. First-in-man evaluation of the transapical APICA ASC™ access and closure device: the initial 10 patients. Eur J Cardiothorac Surg 2013;44:1057-62; discussion 1062. [PubMed]





