Total aortic arch replacement: current approach using the trifurcated graft technique
Introduction
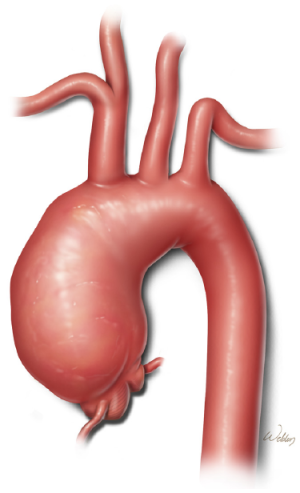
Since the pioneering work of DeBakey, Cooley, and colleagues more than 50 years ago, surgical treatment of aneurysms involving the transverse aortic arch (Figure 1) has been associated with substantial morbidity and mortality. As highlighted throughout this issue of Annals of Cardiothoracic Surgery, many surgeons have dedicated their careers to improving outcomes for patients who undergo these complex procedures. At our center, techniques for replacing the diseased aortic arch have evolved substantially over the past 15 years. Previously, our approach involved femoral cannulation, profound-to-deep hypothermic circulatory arrest and retrograde cerebral perfusion, and the island technique for reattaching the brachiocephalic vessels. In contrast, we currently use innominate artery cannulation, deep-to-moderate hypothermic circulatory arrest with antegrade cerebral perfusion, and the trifurcated graft (Y-graft) technique for reattaching the arch branches (1,2). We have recently described the rationale for these changes in detail (3-5). For those patients who have an arch aneurysm that extends into the descending thoracic aorta, we generally repair the aneurysm by using the elephant trunk technique, which we have also previously described in detail (3,4,6). Many patients, however, have aneurysms that are confined to the ascending aorta and transverse aortic arch, without involvement of the descending thoracic aorta; such patients undergo total aortic arch replacement with the techniques described in this report.
Operative techniques
Exposure and cannulation
We perform aortic arch repair through a standard median sternotomy. Meticulous hemostasis is maintained to reduce problems with bleeding after the aortic repair. We currently favor using the innominate artery as the inflow site for cardiopulmonary bypass. When the innominate artery is not suitable for this purpose because of aneurysm, dissection, or severe atherosclerotic disease involving the vessel, we cannulate the right axillary artery (7). In such cases, access to the axillary artery is obtained through an incision in the right deltopectoral groove, with separation of the pectoralis major muscle fibers, division of the pectoralis minor muscle, and careful mobilization of the adjacent vein and cords of the brachial plexus. Regardless of the inflow access site, we use bilateral cerebral near-infrared spectroscopy sensors to monitor brain oxygenation throughout the procedure.
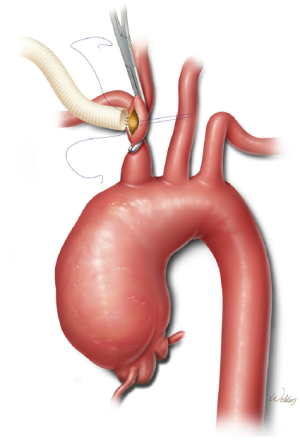
When using the innominate artery as the inflow site, we administer intravenous heparin and apply a partial occluding clamp to the distal aspect of the vessel to achieve both proximal and distal vascular control (Figure 2) (5). After creating a longitudinal arteriotomy, we suture an 8-mm gelatin-sealed, woven polyester graft to the vessel. We use polypropylene sutures for this and all other anastomoses during the procedure. After the graft is carefully flushed and de-aired, it is attached to the inflow line from the cardiopulmonary bypass circuit.
Other cannulas placed in preparation for cardiopulmonary bypass generally include a two-stage venous cannula placed via the right atrium, a left ventricular sump placed via the right superior pulmonary vein, and a coronary sinus retrograde cardioplegia cannula. After cardiopulmonary bypass is initiated, systemic cooling commences to establish deep hypothermia; our target temperature generally ranges between 18 and 23 °C. If the patient has significant aortic valve regurgitation, it is often possible to cross-clamp the ascending aorta to prevent ventricular distension and enable delivery of cardioplegia while maintaining systemic flow and cooling through the innominate artery graft.
Branch vessel reconstruction
During the cooling phase, we begin the process of reconstructing the brachiocephalic vessels. We have found that it is easier to approach the left subclavian artery after the arch aneurysm has been decompressed than during the initial cooling period. Therefore, we generally address the left common carotid artery first and save the left subclavian artery for later. After the mid-portion of the left common carotid artery is clamped and its base is ligated, the artery is divided and sutured end-to-end to the middle branch (which has been stretched and trimmed to an appropriate length to avoid kinking) of a prefabricated trifurcated graft (Figure 3). This anastomosis is performed while full flow from the cardiopulmonary bypass circuit is maintained.
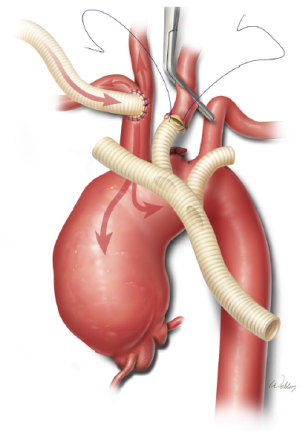
After the left common carotid artery anastomosis is completed and the desired degree of hypothermia has been reached, systemic hypothermic circulatory arrest is established. The proximal aspect of the innominate artery is occluded with a snare, and flow from the pump is reduced to approximately 10 mL/kg/min. The ascending aorta and transverse aortic arch are then opened. To provide perfusion to the left common carotid artery, a balloon-tipped cannula is connected to a Y-limb from the inflow tubing and placed in the proximal aspect of the trifurcated graft (Figure 4). This is particularly important when near-infrared spectroscopy indicates a substantial decline in left brain oxygenation during antegrade perfusion through the right common carotid artery. After the left subclavian artery is transected, the first branch of the trifurcated graft is trimmed to an appropriate length and sutured end-to-end to the vessel. This branch of the graft is de-aired and its clamp is removed, thereby enabling antegrade perfusion to the left subclavian artery.
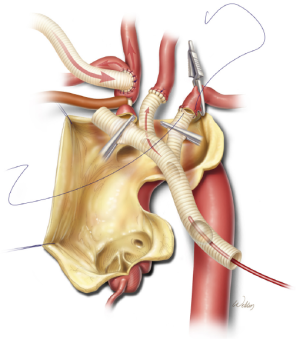
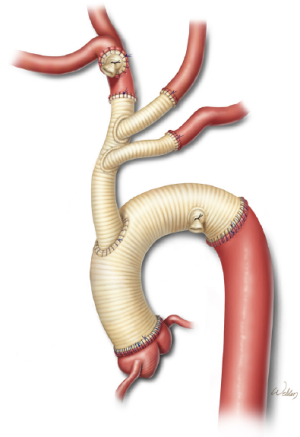
Finally, the distal end of the trifurcated graft is cut to appropriate length and sutured end-to-end to the transected innominate artery (Figure 5). After this anastomosis is completed, the balloon cannula is removed, the innominate artery snare is released, the trifurcated graft is fully de-aired, and the main trunk is clamped (Figure 6). This establishes antegrade cerebral perfusion to all three branches through the innominate inflow graft. Each anastomosis is inspected and selectively reinforced as needed to ensure hemostasis. The trifurcated graft is then reflected in a superior direction, providing unobstructed access to the aortic arch.
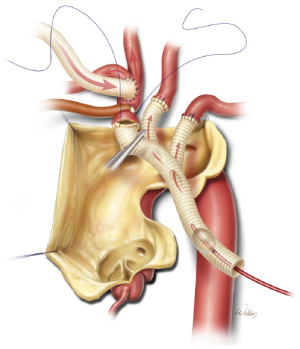
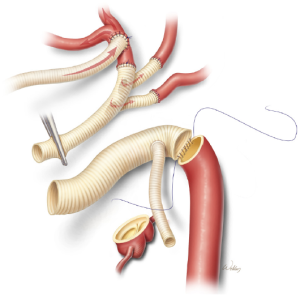
Aortic reconstruction
A gelatin-sealed, woven polyester graft with a suitable diameter and a single side-branch is cut to an appropriate length so that the branch is positioned near the distal end. The distal anastomosis is then created (Figure 6). Our current preferred technique for reinforcing this anastomosis is to run a second continuous suture over the first suture line. Alternatively, individual pledgetted mattress sutures can be placed along the circumference of the anastomosis.
After the distal anastomosis is completed, the inflow Y-limb from the cardiopulmonary bypass circuit is connected to the side-graft. The graft is de-aired and then clamped, which restores distal perfusion (Figure 7). The distal anastomosis is inspected to ensure hemostasis. Attention is then directed to completing the proximal portion of the aortic reconstruction. When this involves an end-to-end anastomosis at the sinotubular junction, we reinforce the suture line with a second running suture or with individual pledgetted mattress sutures.
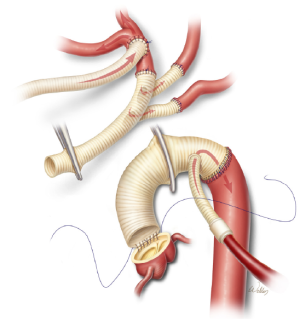
After the proximal anastomosis is completed, an oval opening is created in the right lateral aspect of the ascending aortic graft. It is important to position the opening so that the trifurcated graft will not be kinked and will not be compressed by the sternum after closure. Further, the opening is positioned distal enough to enable safe clamping of the proximal portion of the aortic graft in the event that subsequent cardiac surgery is needed. Gradual rewarming is initiated, and the proximal aspect of the trifurcated graft is cut to an appropriate length in a beveled fashion and sutured to the opening in the aortic graft (Figure 8). The aortic graft is then thoroughly de-aired, and the clamps are removed.
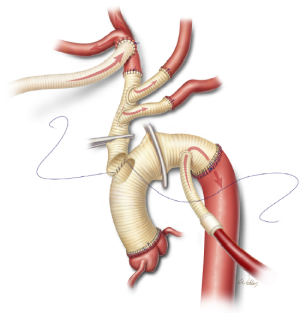
After adequate rewarming and separation from cardiopulmonary bypass, the perfusion grafts in the distal arch and innominate artery are ligated and divided (Figure 9). It is important to use non-absorbable suture to ensure durable occlusion of the transected graft material. The remainder of the procedure, including securing hemostasis and sternotomy closure, proceeds in a standard fashion.
Comments
Our current approach to open total aortic arch repair reflects the incorporation of several new techniques over the past decade. Compared to previous techniques, the recent advances are meant to reduce cerebral ischemia, improve hemostasis, and eliminate residual aortic arch tissue. Early outcomes after aortic arch repair with these techniques have been encouraging, such as those described in our recent retrospective reports (4,5). For example, among 55 patients who recently underwent open total arch repair—including 12 patients (22%) who underwent emergent or urgent operations, 33 (60%) who had a previous sternotomy, and 27 (49%) who underwent a concomitant aortic valve procedure—there was only one (2%) 30-day/in-hospital death (4). Although the median systemic circulatory arrest time was 65 minutes in this cohort, the median cerebral circulatory arrest time was 0 minutes. The median lowest nasopharyngeal temperature was 22.0 °C. Five percent of patients had a stroke, 5% developed renal failure necessitating hemodialysis, and 7% needed reoperation for bleeding. Larger series will be necessary to further evaluate outcomes after these procedures.
Acknowledgements
The authors thank Stephen N. Palmer, PhD, ELS, and Susan Y. Green, MPH, for editorial support. Figures used with the permission of Baylor College of Medicine.
Disclosure: Dr. Coselli serves as a consultant and receives royalties from Vascutek Ltd., a subsidiary of Terumo Corporation.
References
- Spielvogel D, Halstead JC, Meier M, et al. Aortic arch replacement using a trifurcated graft: simple, versatile, and safe. Ann Thorac Surg 2005;80:90-5; discussion 95. [PubMed]
- Spielvogel D, Strauch JT, Minanov OP, et al. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg 2002;74:S1810-4; discussion S1825-32.
- de la Cruz KI, Coselli JS, LeMaire SA. Open aortic arch replacement: a technical odyssey. J Extra Corpor Technol 2012;44:42-7. [PubMed]
- LeMaire SA, Price MD, Parenti JL, et al. Early outcomes after aortic arch replacement by using the Y-graft technique. Ann Thorac Surg 2011;91:700-7; discussion 707-8. [PubMed]
- Preventza O, Bakaeen FG, Stephens EH, et al. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg 2013;145:S191-6. [PubMed]
- LeMaire SA, Carter SA, Coselli JS. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg 2006;81:1561-9; discussion 1569. [PubMed]
- Wong DR, Coselli JS, Palmero L, et al. Axillary artery cannulation in surgery for acute or subacute ascending aortic dissections. Ann Thorac Surg 2010;90:731-7. [PubMed]





