Concomitant atrial fibrillation ablation in minimally invasive cardiac surgery
Introduction
Concomitant atrial fibrillation (AF) ablation during cardiac surgery has shown efficacy in restoring sinus rhythm, with some studies indicating improvement in quality of life and potential reductions in morbidity and even mortality (1,2). Historically, the Cox Maze procedure was the benchmark for AF treatment and has been adapted over time for less invasive cardiac surgeries. A technical description of concomitant ablation techniques in minimally invasive coronary and valvular surgery as performed in our center could offer insights to enhance feasibility, safety, and standardization of practice. Therefore, this paper summarizes our current concomitant AF ablation strategies and techniques.
Techniques such as bipolar epicardial radiofrequency and endo/epicardial cryoablation have evolved as crucial adaptations in concomitant AF treatment, emphasizing the isolation of the atria’s most arrhythmogenic regions. Historically rooted in the Cox Maze procedure, and reinforced by recent studies, pulmonary vein isolation (PVI), left atrial posterior wall isolation and left atrial appendage (LAA) occlusion have emerged as fundamental components of this strategy. While PVI is established as the most rigorously proven effective ablation treatment, especially in paroxysmal AF, it may be insufficient alone for patients with persistent or longstanding-persistent AF. In these cases, additional substrate modification through left atrial posterior wall isolation has shown potential effectiveness (3,4). Furthermore, recent research highlights the critical role of LAA occlusion, not only for stroke risk reduction but also for addressing non-PVI triggers in select patient groups (5-7), thereby solidifying its status as an integral element of concomitant AF treatment.
Although the right atrium has a role in AF, right atrial ablation lines are less frequently performed in minimally invasive procedures for several reasons. Firstly, due to reports of high pacemaker implantation rates (8); secondly, the majority of right atrial lesions originally introduced in the Cox Maze procedure primarily target the prevention of atrial flutter which in most cases can without difficulty be performed by endocardial catheter ablation; and thirdly, the complexity of right atrial ablation lesion set (8). Altogether, the left atrium is the primary focus for concomitant AF surgery in most cases.
However, the evidence remains diverse. Several factors, including patient selection, surgeon’s expertise, and the specific clinical scenario, influence the outcomes. Furthermore, while there is a growing consensus, underscored by guidelines positioning epicardial ablation as a class IIa indication, suggesting its application in most cardiac surgeries, there’s still a pressing need for coherent procedures and guidelines that consider the type of AF and the type ablation set (7,9,10) . Therefore, the Heart Team remains pivotal in the decision-making process, emphasizing individualized considerations based on the patient’s risk factors, the surgery type, and the potential procedure benefits and risks. In the following figures, we will detail our patient selection and step-by-step technique for minimally invasive mitral valve, coronary artery bypass, and aortic valve surgeries, offering a visual insight into our practices.
Operative technique
Patient selection for concomitant AF ablation
Table 1 showcases our Heart Team’s criteria for selecting patients for concomitant AF ablation. This selection process is informed by literature and expert opinions. It includes a decision-making flow chart considering factors such as the type of AF, left atrial volume index, the presence or absence of right atrial dilatation, the type of surgery (whether the left atrium is open or closed), and symptomatology. It’s important to note that our primary focus is on treating patients with symptomatic AF. However, in many cases, it may be challenging to differentiate symptoms of AF from those arising from coronary or valvular heart disease.
Table 1
| Paroxysmal AF | Persistent AF | LSPAF <5 years | LSPAF >5 years | |
|---|---|---|---|---|
| AF symptoms | Yes or no | Yes or no | Yes or no | Yes |
| LAVI <34 mL/m2 | PVI + LAAO | PVI + box + LAAO | PVI + box + LAAO | |
| LA open → + mitral isthmus line | LA open → + mitral isthmus line | |||
| RA dilated → biatrial maze | ||||
| LAVI >34 mL/m2 | PVI + box + LAAO | PVI + box + LAAO | PVI + box + LAAO | |
| LA open → + mitral isthmus line | LA open → + mitral isthmus line | |||
| RA dilated → biatrial maze | ||||
| LAVI >70 mL/m2 | PVI + box + LAAO | No ablation | No ablation | |
AF, atrial fibrillation; LSPAF, longstanding persistent AF; LAVI, left atrial volume index; PVI, pulmonary vein isolation; LAAO, left atrial appendage occlusion; box, left atrial posterior wall; RA, right atrium.
AF ablation in minimally invasive mitral valve surgery
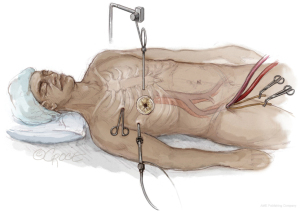
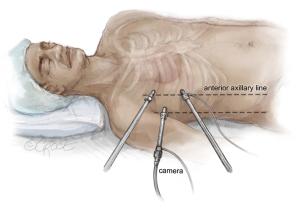
As shown in Figure 1, the patient lies in a supine position. Under echocardiographic guidance, the cardiopulmonary bypass is established through the right femoral vein and artery. We then access the thoracic cavity via a 4-cm thoracotomy in the right 4th intercostal space, using a soft tissue retractor for better visualization. A camera is introduced into the 4th intercostal space. Through a nearby incision, the Chitwood aortic clamp is placed on the aorta, and a cardioplegia cannula is positioned under direct vision in the ascending aorta. With controlled hypotension, the ascending aorta is cautiously clamped, and cardioplegia is administered.
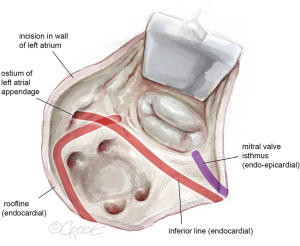
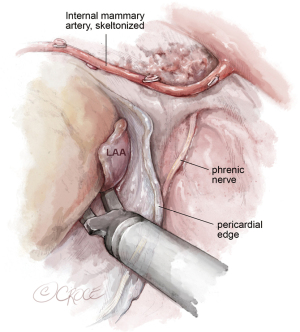
After achieving cardioplegic cardiac arrest, the pericardium is incised below the phrenic nerve. The left atrium is then opened beneath Waterston’s groove, and a left parasternal atrial retractor is placed for better visualization of the left atrium and mitral valve (as shown in Figure 2). The LAA is inspected to ensure it is thrombus-free. Isolation of the pulmonary veins and the posterior left atrial wall is achieved with a box lesion using two endocardial cryo-ablation lines (indicated in red, Figure 2).
The first endocardial cryolesion is running from the atrial incision inferiorly from the inferior pulmonary veins into the LAA (see Figure 3).
The second endocardial cryoline is created by slightly bending the cryoprobe, forming a continuous endocardial lesion from the atrial incision above the superior pulmonary veins, intersecting the first line. It’s vital that these two lines overlap sufficiently.
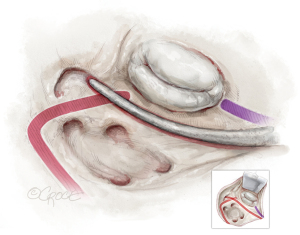
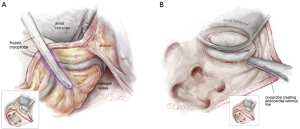
The mitral isthmus line is executed both endo- and epicardially, as shown in Figure 4. The coronary anatomy, whether left-, right-dominant, or balanced, dictates the position of the line. Special care is taken to avoid freezing the posterior descending artery. The epicardial lesion is made first, crossing the coronary sinus and positioning the line between the posterior descending artery and the posterolateral branches (see Figure 4A). Following the epicardial mitral isthmus line, the corresponding endocardial line is set (see Figure 4B and the purple line in Figures 2,4). Care is taken to position the cryoprobe over the mitral annulus, ensuring the endocardial tip is near the posterior mitral valve leaflet.
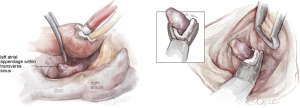
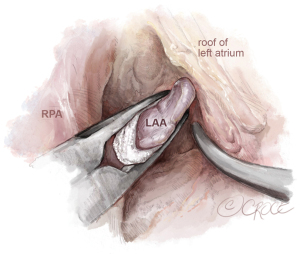
After finalizing the box lesion and mitral isthmus lines, we inspect the LAA using a camera positioned below the aorta through the transverse sinus. We then size and place an endoscopic LAA clip, ensuring the LAA is fully enclosed without any residual pouch. The clip is positioned parallel to the right pulmonary artery, making sure it doesn’t compress the left main coronary artery (see Figure 5). In this figure, a AtriClip V device is shown (Atricure, Ohio, USA), however, in patients with a broader base of the LAA, the fully closed AtriClip PRO2 device may have better control of the far side of the LAA to assure complete closure.
AF ablation in endoscopic coronary bypass surgery
As previously outlined by Verberkmoes et al. (11), the procedure is performed concordantly but with certain modifications. Patients are placed under general anesthesia in a supine position, with both arms resting beside the body to ensure the posterior axillary line remains unobstructed—this is crucial for instrument mobility. Double lumen tube intubation is used for selective lung ventilation. Defibrillator pads are positioned on every patient: one on the left scapula and the other on the right upper abdominal quadrant. A standard transesophageal echocardiography is conducted to inspect the LAA for thrombi.
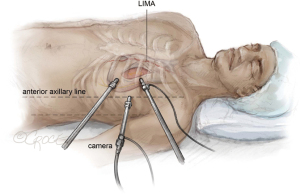
The procedure commences on the left side. For the endoscopic coronary bypass (Endo-CAB) procedure the left side start is crucial for inspection of the left internal mammary artery (LIMA) and coronary targets. A 5-mm trocar is inserted in the mid-axillary line to accommodate a 0-degree 5 mm camera (Karl Storz GmbH, Tuttingen, Germany). A CO2-induced pneumothorax with intrathoracic pressures of 8–12 mmHg is created and both lungs are ventilated during LIMA harvest. In a triangle formation, two additional 5 mm trocars are positioned approximately 3 cm apart from the camera port. The equipment arrangement is depicted in Figure 6. It is worth noting that for optimal LIMA harvesting, the port positioning leans more anterior than the standard stand-alone thoracoscopic AF ablation.
Standard endoscopic tools are utilized. The pericardium is incised at least 2 cm anterior to the phrenic nerve. The target coronary vessels are marked using endoscopic titanium clips. Subsequently, the LIMA is meticulously harvested as detailed by Akca et al. (12). It is important to highlight that the pericardium incision is ventral to the phrenic nerve, diverging from the standard thoracoscopic AF ablation in stand-alone AF. This approach is chosen to accurately locate the left anterior descending artery and ensure accessible anastomosis. After harvesting full length of the LIMA the endoscopic ablation is performed. Heparin is administered after completion of the thoracoscopic AF ablation and the LIMA is not yet divided distally. This to reduce the risk of bleeding during AF ablation and to avoid injury to the LIMA as it lies posteriorly in the field of the AF ablation after dividing distally. After harvesting of the LIMA, selective right lung ventilation is initiated for optimal vision.
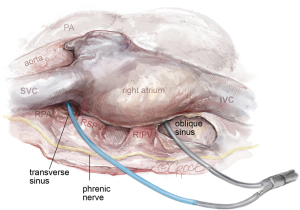
Following this, the oblique sinus is accessed via blunt dissection, positioned between the inferior caval vein and the right lower pulmonary vein. Care is taken to remain just beneath the pulmonary vein plane. Additionally, the transverse sinus is exposed just above the left atrium’s roof, leading into the fat beneath the superior pulmonary vein’s pericardial reflection. The lower trocar is replaced for a 12-mm port and both guiding catheters are inserted into the thoracic cavity through the lower trocar. One is positioned through the oblique sinus, and the other through the transverse sinus, with their tips resting in the pericardial cavity on the right side.
The procedure then progresses to the right side, utilizing single left lung ventilation, as illustrated in Figure 7. The trocars are situated in identical intercostal spaces as on the left, albeit slightly more posterior to enhance instrument angulation.
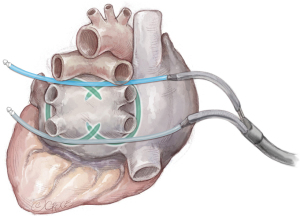
The pericardium is incised 2 cm above of the phrenic nerve. Fat in Waterston’s groove is divided using diathermy. Both guides are retrieved and routed through the 12 mm right lower trocar. The bipolar RF clamp (Gemini S, Medtronic) attaches to the guides, with its concave side facing the heart, as depicted in Figure 8. It is then positioned around the pulmonary veins, and bipolar ablation is executed five times from the right.
The procedure is again continued on the left side and clamping is also performed with 5× bipolar ablation with the concave site of the clamp towards the heart completing the box (see Figures 9,10). Visual overlap of the bilateral ablation lines is assessed trough the oblique sinus and after electrical cardioversion to sinus rhythm (when patient is still in AF), sensing and pacing is performed to confirm entrance and exit block.
A clip to the LAA is placed through the 12 mm lower trocar (not shown in figures) in all patients (AtriClip PRO2, Atricure, Mason, Ohio, USA). After completion of the ablation procedure and clip placement, heparin is administered and the LIMA is clipped and divided distally. The procedure is continued with the off-pump endo-CAB as described earlier (12).
LAA closure in mini-AVR
Aortic valve replacement via mini-sternotomy is carried out using a J-incision in the third intercostal space. However, conducting pulmonary vein or box ablation through this method has proven challenging with the current available devices. Consequently, all patients requiring aortic valve surgery and exhibiting symptomatic AF, undergo surgery via a standard sternotomy. Concurrent AF ablation is carried out in accordance with the flowchart shown in Table 1. Meanwhile, patients with asymptomatic AF who need aortic valve surgery receive treatment using the mini-AVR method, which includes closure of the LAA, as illustrated in Figure 11 through the transverse sinus (also depicted in Figure 5). Alternatively, the atrial clip can be placed (after clamping the aorta) directly lateral left from the aorta and pulmonary artery (not shown).
Comments
The shift to less invasive techniques in AF treatment balances the need for effective therapy with patient safety. Our approach targets critical areas like the pulmonary veins, left atrial posterior wall, and LAA, crucial for AF management. We selectively bypass more complex ablation sites to reduce risk and aid quicker recovery. This strategy effectively tackles primary AF causes, merging the depth of traditional surgery with the advantages of minimally invasive methods. In minimally invasive cardiac surgery, ablation tools are vital for AF treatment. However, these tools need continual adaptions by new innovations, especially in mini-AVR procedures, where accessing pulmonary veins and the left atrial posterior wall is challenging. Possible solutions to continue minimally invasive surgery and combine AF treatment may be the development of new epicardial devices, or to introduce a hybrid approach. As shown by stand-alone AF treatments, a hybrid approach can optimize outcomes (3,13). Hybrid procedures combining surgical and catheter-based ablations can address practical and quality challenges in concomitant ablation during minimally invasive cardiac surgery. Starting with surgical steps like LAA clipping and PVI, followed by catheter ablations, this method has the potential to comprehensively treat complex AF cases and maintain minimally invasiveness for the primary surgical procedure. Future studies are needed to evaluate these options.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: F.A., T.J.B. and N.V. are proctor for Medtronic. The authors have no other conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kowalewski M, Pasierski M, Kołodziejczak M, et al. Atrial fibrillation ablation improves late survival after concomitant cardiac surgery. J Thorac Cardiovasc Surg 2023;166:1656-1668.e8. [Crossref] [PubMed]
- Maesen B, van der Heijden CAJ, Bidar E, et al. Patient-reported quality of life after stand-alone and concomitant arrhythmia surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2022;34:339-48. [Crossref] [PubMed]
- van der Heijden CAJ, Weberndörfer V, Vroomen M, et al. Hybrid Ablation Versus Repeated Catheter Ablation in Persistent Atrial Fibrillation: A Randomized Controlled Trial. JACC Clin Electrophysiol 2023;9:1013-23. [Crossref] [PubMed]
- Harlaar N, Oudeman MA, Trines SA, et al. Long-term follow-up of thoracoscopic ablation in long-standing persistent atrial fibrillation. Interact Cardiovasc Thorac Surg 2022;34:990-8. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Hocini M, Shah AJ, Nault I, et al. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm 2011;8:1853-61. [Crossref] [PubMed]
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024;149:e1-e156. Erratum in: Circulation 2024;149:e167. [Crossref] [PubMed]
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399-409. [Crossref] [PubMed]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275-444. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. Erratum in: Eur Heart J 2021;42:507 Erratum in: Eur Heart J 2021;42:546-7. Erratum in: Eur Heart J 2021;42:4194. [Crossref] [PubMed]
- Verberkmoes N, Olsthoorn J, Dekker L. Minimally invasive totally thoracoscopic stand-alone surgical ablation for atrial fibrillation. Multimed Man Cardiothorac Surg 2020;2020: [Crossref] [PubMed]
- Akca F, Ter Woorst J. Learning Curve of Thoracoscopic Nonrobotic Harvest of the Left Internal Mammary Artery in Minimally Invasive Coronary Artery Bypass Grafting. Innovations (Phila) 2023;18:262-5. [Crossref] [PubMed]
- Doll N, Weimar T, Kosior DA, et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: a randomised, controlled trial. EClinicalMedicine 2023;61:102052. [Crossref] [PubMed]