The natural history of aortic root aneurysms
Introduction
Our team at the Yale Aortic Institute has been studying the natural history of ascending and descending thoracic aortic aneurysm for more than three decades (1-13). Our early studies provided evidence-based criteria regarding the appropriate aortic size for elective prophylactic intervention on the aorta (14). However, those studies included only several hundred patients (our early experience) and could not provide adequate granularity to permit separating patients into the “ascending” and “descending” groups. Since then, as our database has grown to over 4,000 patients with various aortic pathologies, the increased clinical data has provided the ability to scrutinize the data in much finer detail, permitting accurate analysis for each specific segment of the aorta. In this regard, the aortic root deserves special attention, being most proximally in line to bear the full strength of the left ventricular stroke volume, having a unique cloverleaf anatomical configuration, and also, quite critically, giving rise to the coronary arteries. In this manuscript and accompanying lecture, we review the natural history of ascending aortic aneurysm with a focus on what is currently known specifically regarding the aortic root.
Why is the aortic root different?
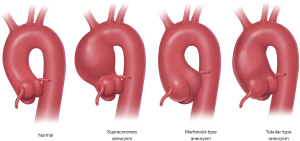
It is well-recognized that aneurysms of the ascending aorta occur in various anatomic configurations (15) (Figure 1). Some aneurysms involve only the supracoronary aorta and spare the aortic root. In other cases, the aneurysm involves only the aortic root (as is typical for Marfan syndrome, for example) and spares the supracoronary aorta. Finally, some patients share characteristics, combining to form a more diffuse “tubular” generalized enlargement that involves both the root and the ascending portions.
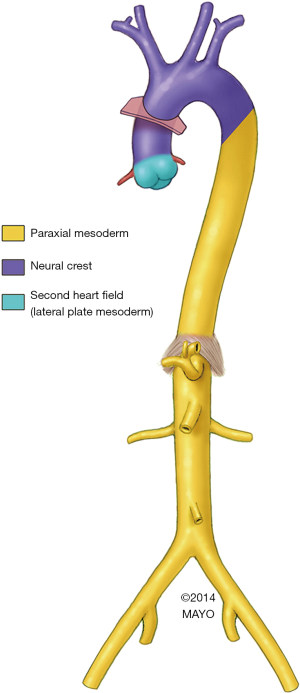
Embryology may underlie the anatomic patterns. We now know that much of the ascending aorta and aortic arch develops from the cardiac neural crest (Figure 2) (16,17). However, specifically the aortic root portion of the ascending aorta is derived primarily from the second heart field (16,18). Of note, the descending aorta too has its own embryologic source, developing from the mesoderm, which likely underlies the marked differences we have noted between the clinical presentation and behavior of ascending and descending aortic aneurysms (19).
Natural history of ascending aortic aneurysm
The typical natural history of ascending aortic aneurysm is shown in Figure 3. The disease-prone aorta [typically genetically mediated (20,21)] dilates slowly, growing at a rate of ~1–2 mm/year (9,10). Please note that as the aorta enlarges, its rate of growth also increases. This is important in deciding the timing of intervention in large-size ascending aneurysms. As the aorta reaches a critical size threshold, a sudden dramatic increase in blood pressure [often precipitated by extreme emotion or exertion (22)] causes the aortic wall to experience levels of mechanical stress that exceed its tensile limits (800–1,000 kPa) (23), causing it to dissect. The dissection is believed to be initiated by a tear in the intimal layer of the aorta, which allows blood to enter the medial layer and create two lumens for blood flow. Unless emergent surgical treatment is readily available, the patient is likely to die, with mortality risk increasing by 0.5% per hour [according to the most recent data from International Registry of Acute Aortic Dissection (IRAD) (24)].
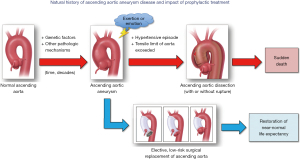
Based on this sobering outlook of ascending aortic aneurysm (if left untreated), our main goal is to identify patients at risk in time (before the cataclysmic event of aortic dissection) and to perform prophylactic elective surgery to prevent that outcome. Identification of patients with ascending aortic aneurysm is a challenge in and of itself due to its asymptomatic nature. Collecting accurate family history information (25,26), genetic testing of first- and second-degree relatives (27), imaging individuals with positive family histories and/or positive genetic findings (28), and utilizing the “Guilt-by-Association” paradigm (29), are all means to identify individuals who may be harboring a symptomless aneurysm in their chest.
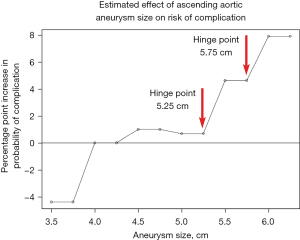
Once the individual with an ascending aortic aneurysm has been identified, the question becomes—how to determine the optimal time to conduct the prophylactic surgery? Aortic size (diameter) has been shown to be the most important predictor of adverse outcome. By analyzing thousands of patients in our Aortic Institute database and plotting the risk of aortic rupture and dissection against the size of the ascending aorta, we were able to identify two “hinge-points”—at 5.25 and 5.75 cm—at which the risk of aortic adverse events increases dramatically (see Figure 4) (10), signifying the need for prophylactic surgery before the ascending aorta reaches those critical sizes.
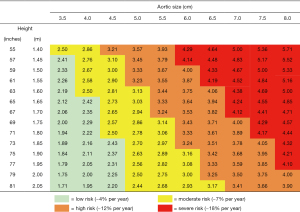
Historically, we and others have recommended ascending aortic resection when the aorta reaches 5.5 cm (and 5.0 cm for patients with Marfan syndrome) (30,31). However, in recent years new data have emerged suggesting that intervention at a somewhat smaller ascending aortic size is more protective against sudden events (32,33). On this basis, in recent years, we have recommended a “left-shift” to an earlier general criterion for intervention. In the newest 2022 iteration of the Guidelines on the Management of Aortic Disease, the traditional criterion has been revised to 5.0 cm (with even smaller size thresholds recommended for patients with connective tissue disorders) (34). It is important to note that one size does not necessarily “fit all” when it comes to decision-making regarding prophylactic ascending aortic resection. Indexing the size of the ascending aorta to a person’s height provides a more granular, more precise prediction of risk of aortic events in individuals of different stature (see chart in Figure 5) (10). This breakdown is especially useful for individuals at the extremes of body size (very short or very tall).
Of course, it goes without saying that a symptomatic ascending or aortic root aneurysm needs resection, almost regardless of size. Pain is the only avenue by which the jeopardized aorta can communicate with us (35,36).
Natural history of aortic root aneurysm
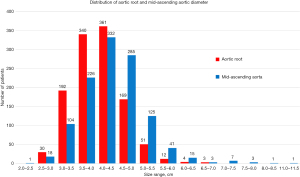
To investigate the natural history of aneurysms involving the aortic root (with or without the ascending aorta), we conducted a study (37) in 1,162 patients, all of whom had high-quality computer tomography (CT) or magnetic resonance imaging (MRI) images available for dedicated re-analysis of ascending aortic and aortic root size. Figure 6 illustrates the frequency distribution of aortic root and mid-ascending aortic aneurysms by aortic size. At smaller sizes, the proportion of aortic root aneurysm is higher, and conversely the proportion of mid-ascending aortic aneurysm is higher at larger aortic sizes. This difference is also confirmed by the mean aortic size values, which are 4.02±0.60 and 4.33±0.77 cm for aortic root and mid-ascending aortic aneurysms, respectively.
We evaluated the following specific end-points: Type A dissection (n=120), ascending aortic rupture without antecedent dissection (n=2), confirmed ascending aortic death (n=8), and all other causes of death (n=119). Of the remaining patients in the study cohort, 545 underwent prophylactic surgical management for their root/ascending aortic aneurysm. However, as has been our policy for many years, we did not use “surgery” as an endpoint for natural history calculations, since the decision to move forward with elective surgery is purely based on the surgeon’s decision and may not be a fair representation of the underlying natural history of the disease.
In plotting the lifetime risk of aortic root and mid-ascending aortic aneurysm against aortic size (Figure 7), we see that for the aortic root the risk starts to increase substantially immediately after reaching 5.0 cm, while for the mid-ascending aorta the risk increases after 5.25–5.5 cm (note the inflection point where each curve starts upward). In a Cox regression analysis of these data, aortic root aneurysm emerged as a significant risk factor associated with the studied endpoints of aortic events and death. These data signify that aortic root dilatation is more malignant and dangerous than dilatation solely of the supracoronary segment of the ascending aorta.

With knowledge of this differing outlook for aortic root and supracoronary mid-ascending aneurysm, we hypothesize that the two distinct “hinge-points” that we had identified in our prior studies (see Figure 4) could potentially be explained by the different locations of disease in the ascending aorta, wherein the smaller hinge-point corresponds to risk conferred by the aortic root, while the larger hinge-point shows the risk of root-sparing mid-ascending aortic aneurysm.
Genetic predisposition contributes to malignant aortic root disease
Since the discovery of the familial nature of thoracic aortic disease in the late 1990s (25,26), major strides have been made towards understanding the clinical and molecular genetics of this disease. One out of every five patients with thoracic aortic disease has at least one other first-degree relative with some form of aortopathy or aneurysm-related disease (25,26). Most familial cases of aortic disease are inherited in an autosomal dominant fashion (38), so that in large families every generation will typically have at least one affected individual. Thoracic aortic diseases are subdivided into syndromic (with extra-aortic features) and non-syndromic (with disease limited to the aorta) cases, with the latter category being further subdivided into familial and sporadic cases. Although patients with syndromic thoracic aortic disease (such as Marfan, Loeys-Dietz, Ehlers-Danlos, and Turner’s syndromes) can present with aortic aneurysm at various locations, the aortic root specifically appears to be the most common site for aortic dilatation (39-41). Such root dilatation in syndromic cases is typically very malignant, as it develops early in life (even during childhood) and leads to aortic dissection or rupture at smaller aortic sizes and younger ages than typical non-syndromic patients.
Although clinical identification of patients with syndromic aortopathies is somewhat easier (than non-syndromic patients) due to the multitude of extra-aortic manifestations and its common familial nature, molecular genetics have become increasingly important over the past decade for diagnosis, confirmation, and familial screening for both syndromic and non-syndromic thoracic aortic disease (20). To date, more than 70 genes have in some way been implicated in thoracic aortic disease, although only 24 of these genes have been confirmed by ClinGen (with 11 genes classified as strong/definitive, 4 genes as moderate, and 9 genes as limited evidence of causation) (42).
Interestingly, a change in only one single nucleotide—only one of the 3.2 billion “letters” making up the human genome—in one of these known risk genes is sufficient to cause familial thoracic aortic aneurysm disease. This is simply remarkable—as if a single grain of sand determined the fate of a huge beach biosphere. Routine clinical genetic testing via exome sequencing (27) currently has become a cost-effective way to test for pathogenic variants in the currently known risk genes. As the sequencing data is held permanently, this also permits re-visiting the data later to check for variants in any newly discovered causative genes.
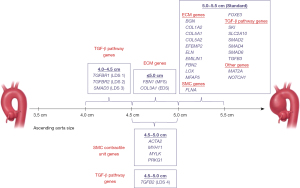
Another reason why genetic testing is important is that mutations of some genes modify the “typical” natural history of thoracic aortic aneurysm disease, rendering the disease more malignant. For example, mutations in the ACTA2 gene cause aortic dissection at sizes significantly smaller than even the current guideline-driven general intervention criteria on the ascending aorta (43-45). Similarly, mutations in the MYLK gene are almost exclusively implicated in aortic dissection, which can occur in near-normal-sized aortas (46,47). Although counseling patients who harbor these mutations regarding the appropriate timing (or aortic size) for prophylactic intervention is challenging, it is much preferable to not being aware of the dangerous mutation. Absent that knowledge, the clinical scenario would likely lead to sudden aortic event and a high likelihood of death. To assist clinicians in determining the most appropriate timing for surgical intervention, we provide a chart that plots the causative genes, indicating specific aortic sizes, at which prophylactic surgery is recommended (see Figure 8) (48).
In the current era of widespread and continuously growing access to genomic sequencing technologies for clinical diagnostic testing, some challenges remain in the field of thoracic aortic disease. One of the most significant challenges has to do with the fact that only about 3–4% of variants in known risk genes for thoracic aortic disease are classified as “pathogenic” or “likely pathogenic” [according to criteria of the American College of Medical Genetics and Genomics (ACMG) (49)]. The remaining 25–30% of variants in these known risk genes are classified as “variants of uncertain significance” (VUS) (27). This means that there is simply not enough conclusive evidence to classify these variants either as completely benign or definitively pathogenic. This is problematic, because many of these variants, although suspicious for being disease-causing (based on population variant frequency, conservation in phylogeny, in silico prediction of effect on protein structure, etc.), do not entirely satisfy the very strict ACMG criteria. This conundrum is prone to creating some friction between the clinical geneticist and the cardiac surgeon, with the former striving for scientifically accurate guideline-driven variant curation and the latter aiming to protect the patient from developing aortic complications. Certainty of association is best confirmed by noting correlation of genotype with phenotype over generations. However, since such correlation requires multiple decades, this is largely impractical and not useful for clinical care of a specific patient. In searching for ways to rapidly test the potential effect of a specific VUS on the aorta, we are currently developing a zebrafish model, which, in preliminary investigations, has shown promise for evaluating such genomic variants quickly and, we hope, effectively (50).
Safety of preserving the aortic root during ascending aortic surgery
Another aspect of evaluating the natural history of the aortic root is to determine what happens to the root in the long-term after root-sparing procedures. Such procedures are usually performed in patients with non-syndromic aortic aneurysm since syndromic manifestation of this disease frequently involves the aortic root.
In patients undergoing elective ascending aortic aneurysm surgery with only a mildly dilated aortic root, some surgeons may be inclined to leave the aortic root untouched, limiting the operation to replacement of only the supracoronary segment of the aorta (with or without intervention on the aortic valve). However, this raises valid concerns about whether the native aortic root will eventually dilate over time and require a potentially dangerous reoperation. Sparing the root may be especially attractive in elderly or infirm patients. We studied this in 102 patients with non-syndromic ascending aortic disease who underwent elective root-sparing procedures (51). The mean postoperative baseline aortic root diameter was 37.4±3.76 mm (range, 27–48 mm). The mean growth rate of the retained aortic root was 0.41 mm/year, significantly lower than the typical rate of aortic aneurysm growth (1–2 mm/year). During a mean follow-up of 6 years (range, 1–12 years), no patient required replacement of the primarily untouched root or suffered dissection of the proximal aorta. Freedom from aortic root events (aortic root replacement, aneurysm, or dissection of the untouched root) was 100% at 1, 5, and 10 years (51). The study also found that the average 3.7 cm aortic root in the average 62-year-old patient would require 29 years to reach the currently accepted 5.0 cm threshold for intervention. Only patients with initial aortic root sizes of 4.5 cm or greater were found to be at any potential risk of subsequent aortic events.
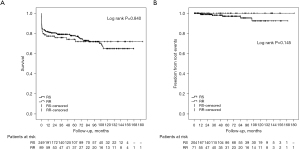
In patients undergoing surgery for acute Type A aortic dissection, the extent of aortic resection has been an enduring matter of debate (both proximally and distally). Many surgeons would prefer the relative “simplicity” of leaving the non- or minimally dilated root behind, but this is balanced against the worry about the fate of the spared aortic root thereafter. We studied the outcomes of sparing the root in 249 Type A dissection patients (52) and found that the post-surgery growth rate was similar to what we saw in spared roots in the absence of aortic dissection (0.4 cm/year on average). The long-term survival and freedom from aortic root events were not statistically significant between patients undergoing root replacement and those having root sparing procedures (see Figure 9). Although the spared roots often appeared irregular, distorted, and enlarged, only seven patients (3%) in the root-sparing group suffered root events and required some type of intervention on either the aortic root or the aortic valve (or both) (52). Based on these data, we feel that sparing the non-dilated root is safe in the setting of an acute Type A dissection, with low secondary root events or re-interventions.
Safety of aortic root surgery in the present era
Over the past three decades, surgical risk during elective operations on the ascending aorta has become very low, allowing wide flexibility in decision to operate and the extent of resection. In the early years of aortic root surgery, high risk of the operation itself led to appropriate reluctance to operate, except for the largest aneurysms, which were already known to carry very high risk. Currently, with risks as low as in Table 1, surgery on the ascending aorta and aortic root is approaching a safety similar to “routine” coronary artery bypass grafting, long considered the most common, “standard” open heart procedure.
Table 1
| Reference | Location of aortic surgery | Operative mortality | Postoperative stroke |
|---|---|---|---|
| Mok et al. 2017, (53) | Composite graft aortic root replacement | 1.9% | 1.4% |
| Peterss et al. 2016, (54) | Root-sparing ascending aortic replacement | 0% | 1.0% |
| Ziganshin et al. 2014, (55) | Aortic arch replacement with DHCA | 1.4% | 1.2% |
DHCA, deep hypothermic circulatory arrest.
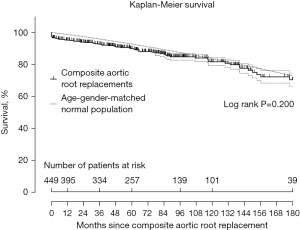
We investigated the safety of composite graft aortic root replacement (both mechanical and biological) in a study encompassing 25 years of clinical experience and found that this procedure produces a long-term outlook that matches the life-expectancy of an age- and gender-matched general population (53) (see Figure 10 and also schematic in Figure 3). Freedom from bleeding and thromboembolism was 99%, 98%, 95%, 94% and 94% at 1, 5, 10, 15, and 20 years, respectively (53). Freedom from late reoperation on the aortic root was 99.5%, 99%, 99%, 98%, and 98%, at 1, 5, 10, 15, and 20 years, respectively (53). Valve-sparing operations, as well, have become standardized and very safe in the current era in experienced hands, leading to excellent long-term outcomes, as detailed in dedicated papers in this issue.
Conclusions
In conclusion, aortic root dilation, common in genetically mediated syndromic thoracic aortic disease, is a more malignant and dangerous entity than other ascending aortic aneurysms that do not involve the aortic root. Genetic testing via exome sequencing is recommended for patients with root aneurysm to rule out genes that confer a more malignant natural history course of the disease. Sparing the non-dilated aortic root during ascending aneurysmectomy is safe, even in the setting of acute Type A aortic dissection, unless it is larger than 4.5 cm. Finally, replacement of the aortic root in experienced centers produces excellent results that restore normal life expectancy for the patient.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elefteriades JA, Hartleroad J, Gusberg RJ, et al. Long-term experience with descending aortic dissection: the complication-specific approach. Ann Thorac Surg 1992;53:11-20; discussion 20-1. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 1997;113:476-91; discussion 489-91. [Crossref] [PubMed]
- Rizzo JA, Coady MA, Elefteriades JA. Procedures for estimating growth rates in thoracic aortic aneurysms. J Clin Epidemiol 1998;51:747-54. [Crossref] [PubMed]
- Elefteriades JA, Lovoulos CJ, Coady MA, et al. Management of descending aortic dissection. Ann Thorac Surg 1999;67:2002-5; discussion 2014-9. [Crossref] [PubMed]
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002;74:S1877-80; discussion S1892-8. [Crossref] [PubMed]
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169-77. [Crossref] [PubMed]
- Davies RR, Kaple RK, Mandapati D, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 2007;83:1338-44. [Crossref] [PubMed]
- Elefteriades JA, Meier P. Clopidogrel and cardiac surgery: enemy or friend? Heart 2012;98:1685-6. [Crossref] [PubMed]
- Elefteriades JA, Ziganshin BA, Rizzo JA, et al. Indications and imaging for aortic surgery: size and other matters. J Thorac Cardiovasc Surg 2015;149:S10-3. [Crossref] [PubMed]
- Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg 2018;155:1938-50. [Crossref] [PubMed]
- Wu J, Zafar MA, Li Y, et al. Ascending Aortic Length and Risk of Aortic Adverse Events: The Neglected Dimension. J Am Coll Cardiol 2019;74:1883-94. [Crossref] [PubMed]
- Zafar MA, Chen JF, Wu J, et al. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2021;161:498-511.e1. [Crossref] [PubMed]
- Chen JF, Zafar MA, Wu J, et al. Increased Virulence of Descending Thoracic and Thoracoabdominal Aortic Aneurysms in Women. Ann Thorac Surg 2021;112:45-52. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Elefteriades JA. Developing surgical intervention criteria for thoracic aortic aneurysms. Cardiol Clin 1999;17:827-39. [Crossref] [PubMed]
- Elefteriades JA, Ziganshin BA. Practical Tips in Aortic Surgery: Clinical and Technical Insights. Switzerland: Springer, 2021.
- Maleszewski JJ. Inflammatory ascending aortic disease: perspectives from pathology. J Thorac Cardiovasc Surg 2015;149:S176-83. [Crossref] [PubMed]
- Jiang X, Rowitch DH, Soriano P, et al. Fate of the mammalian cardiac neural crest. Development 2000;127:1607-16. [Crossref] [PubMed]
- Cheung C, Bernardo AS, Trotter MW, et al. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol 2012;30:165-73. [Crossref] [PubMed]
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010;55:841-57. [Crossref] [PubMed]
- Ostberg NP, Zafar MA, Ziganshin BA, et al. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020;10:182. [Crossref] [PubMed]
- Pinard A, Jones GT, Milewicz DM. Genetics of Thoracic and Abdominal Aortic Diseases. Circ Res 2019;124:588-606. [Crossref] [PubMed]
- Hatzaras IS, Bible JE, Koullias GJ, et al. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol 2007;100:1470-2. [Crossref] [PubMed]
- Koullias G, Modak R, Tranquilli M, et al. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. J Thorac Cardiovasc Surg 2005;130:677-83. [Crossref] [PubMed]
- Harris KM, Nienaber CA, Peterson MD, et al. Early Mortality in Type A Acute Aortic Dissection: Insights From the International Registry of Acute Aortic Dissection. JAMA Cardiol 2022;7:1009-15. [Crossref] [PubMed]
- Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg 1999;134:361-7. [Crossref] [PubMed]
- Biddinger A, Rocklin M, Coselli J, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg 1997;25:506-11. [Crossref] [PubMed]
- Ziganshin BA, Bailey AE, Coons C, et al. Routine Genetic Testing for Thoracic Aortic Aneurysm and Dissection in a Clinical Setting. Ann Thorac Surg 2015;100:1604-11. [Crossref] [PubMed]
- Elefteriades JA, Mukherjee SK, Mojibian H. Discrepancies in Measurement of the Thoracic Aorta: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:201-17. [Crossref] [PubMed]
- Ziganshin BA, Elefteriades JA. Guilt by association: a paradigm for detection of silent aortic disease. Ann Cardiothorac Surg 2016;5:174-87. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Ziganshin BA, Zafar MA, Elefteriades JA. Descending threshold for ascending aortic aneurysmectomy: Is it time for a "left-shift" in guidelines? J Thorac Cardiovasc Surg 2019;157:37-42. [Crossref] [PubMed]
- Elefteriades JA, Rizzo JA, Zafar MA, et al. Ascending aneurysmectomy: Should we shift to the left? J Thorac Cardiovasc Surg 2022;S0022-5223(22)00833-9.
- Writing Committee Members. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;80:e223-393. [PubMed]
- Elefteriades JA, Tranquilli M, Darr U, et al. Symptoms plus family history trump size in thoracic aortic aneurysm. Ann Thorac Surg 2005;80:1098-100. [Crossref] [PubMed]
- Papanikolaou D, Zafar MA, Tanweer M, et al. Symptoms Matter: A Symptomatic but Radiographically Elusive Ascending Aortic Dissection. Int J Angiol 2019;28:31-3. [Crossref] [PubMed]
- Kalogerakos PD, Zafar MA, Li Y, et al. Root Dilatation Is More Malignant Than Ascending Aortic Dilation. J Am Heart Assoc 2021;10:e020645. [Crossref] [PubMed]
- Albornoz G, Coady MA, Roberts M, et al. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg 2006;82:1400-5. [Crossref] [PubMed]
- Judge DP, Dietz HC. Marfan's syndrome. Lancet 2005;366:1965-76. [Crossref] [PubMed]
- MacCarrick G, Black JH 3rd, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med 2014;16:576-87. [Crossref] [PubMed]
- Wenstrup RJ, Meyer RA, Lyle JS, et al. Prevalence of aortic root dilation in the Ehlers-Danlos syndrome. Genet Med 2002;4:112-7. [Crossref] [PubMed]
- Renard M, Francis C, Ghosh R, et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J Am Coll Cardiol 2018;72:605-15. [Crossref] [PubMed]
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007;39:1488-93. [Crossref] [PubMed]
- Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet 2009;84:617-27. [Crossref] [PubMed]
- Regalado ES, Guo DC, Prakash S, et al. Aortic Disease Presentation and Outcome Associated With ACTA2 Mutations. Circ Cardiovasc Genet 2015;8:457-64. [Crossref] [PubMed]
- Wang L, Guo DC, Cao J, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 2010;87:701-7. [Crossref] [PubMed]
- Regalado ES, Morris SA, Braverman AC, et al. Comparative Risks of Initial Aortic Events Associated With Genetic Thoracic Aortic Disease. J Am Coll Cardiol 2022;80:857-69. [Crossref] [PubMed]
- Faggion Vinholo T, Brownstein AJ, Ziganshin BA, et al. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2019 Update and Clinical Implications. Aorta (Stamford) 2019;7:99-107. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Prendergast A, Ziganshin BA, Papanikolaou D, et al. Phenotyping Zebrafish Mutant Models to Assess Candidate Genes Associated with Aortic Aneurysm. Genes (Basel) 2022;13:123. [Crossref] [PubMed]
- Peterss S, Bhandari R, Rizzo JA, et al. The Aortic Root: Natural History After Root-Sparing Ascending Replacement in Nonsyndromic Aneurysmal Patients. Ann Thorac Surg 2017;103:828-33. [Crossref] [PubMed]
- Peterss S, Dumfarth J, Rizzo JA, et al. Sparing the aortic root in acute aortic dissection type A: risk reduction and restored integrity of the untouched root. Eur J Cardiothorac Surg 2016;50:232-9. [Crossref] [PubMed]
- Mok SC, Ma WG, Mansour A, et al. Twenty-five year outcomes following composite graft aortic root replacement. J Card Surg 2017;32:99-109. [Crossref] [PubMed]
- Peterss S, Charilaou P, Dumfarth J, et al. Aortic valve disease with ascending aortic aneurysm: Impact of concomitant root-sparing (supracoronary) aortic replacement in nonsyndromic patients. J Thorac Cardiovasc Surg 2016;152:791-798.e1. [Crossref] [PubMed]
- Ziganshin BA, Rajbanshi BG, Tranquilli M, et al. Straight deep hypothermic circulatory arrest for cerebral protection during aortic arch surgery: Safe and effective. J Thorac Cardiovasc Surg 2014;148:888-98; discussion 898-900. [Crossref] [PubMed]






