Continuous-flow left ventricular assist device systems infections: current outcomes and management strategies
Introduction
Heart failure is one of the leading causes of morbidity and mortality in the United States, with increasing hospitalization rates as our population ages (1,2). Advanced heart failure management is challenging, necessitates frequent admissions, and is associated with high cost of care (1). Heart transplantation is a preferred management option for eligible patients with advanced heart failure. The number of patients actively awaiting heart transplant has increased in recent years, with continued increase in the proportion of heart transplant candidates who are sixty-five years or older (3). However, available organs remain limited. Therefore, left ventricular assist devices (LVADs) have emerged as an attractive alternative option for management of advanced heart failure, and have been increasingly used as both as a bridge to transplantation, as well as destination therapy.
LVADs offer an option for managing heart failure symptoms and improving patients’ quality of life, however complications associated with the devices are substantial. Infection remains one of the most common adverse events reported in patients with mechanical circulatory support (MCS). The international society for heart and lung transplantation (ISHLT) mechanically assisted circulatory support (IMACS) registry published an analysis of adverse outcomes during the time period of 2013−2015, with infection reported as the most common adverse event—37% of all patients with MCS, followed by bleeding (33%), stroke (18%), respiratory failure (17%) and device malfunction in 12% of patients (4). The most common type of infection was non-VAD infections, 66%, occurring early post implantation, usually less than three months. VAD-specific infections were the second most common group, out of which the most common was driveline infections (82.9%). In this report, the mean time from device implant to VAD-specific infection was around seven months.
LVAD infections are classified as LVAD-specific, LVAD-related and non-LVAD related infections (5) (Table 1).

Full table
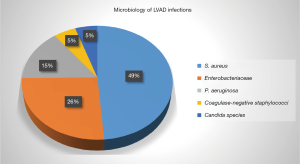
LVAD-specific infection can be introduced directly during placement of an LVAD or can develop later as driveline exit site infection, pump/pocket infection as an extension of driveline infection, or hematogenous seeding of the device from non LVAD associated infection. The most commonly reported organisms associated with LVAD infection are gram-positive bacteria, followed by gram-negative infections. Fungi are much less common (<10%). Among gram positive bacteria, S. aureus and S. epidermidis are the most commonly identified organisms (6) (Figure 1).
When evaluating patients for possible LVAD infection, the patient’s risk factors for infection should be considered. Older studies evaluating risk factors for LVAD infections were retrospective cohort studies of pulsatile flow devices. Newer studies evaluating the risk of MCS infections in continuous-flow LVADs (CF-LVAD) list higher BMI, younger age, and exposed driveline velour as risk factors for LVAD driveline infections (7). Driveline trauma is a commonly reported risk factor for development of subsequent LVAD infection. This includes accidental trauma from pulling and/ or disruption of the seal between the skin and the driveline (8). Of note, the location of the driveline exit site; chest wall vs. abdomen, was not found to be associated with decreased risk of infection (9).
One retrospective multicenter observational study from France reported increased risk for LVAD infections in patients with HeartMate II LVAD (compared to HeartWare HVAD and Jarvik 2000), and in patients requiring implantable cardioverter defibrillator (ICD) related procedures after the LVAD surgery (6). This finding differs from the findings of the ENDURANCE trial, which showed no difference in infection rates between HeartMate II LVAD and HeartWare HVAD (10).
Diagnosis
Accurate diagnosis and classification of infections in LVAD recipients as LVAD-specific, LVAD-related, or non-LVAD related is crucial for optimal management. Most driveline infections can be diagnosed based on clinical manifestations of infection such as erythema, pain, tenderness, or drainage. However, imaging such as computed tomography (CT) scan of the chest/abdomen/pelvis may be necessary to diagnose deep driveline infections or pump/cannula infections. Accurately classifying bloodstream infections in LVAD recipients can be challenging. Transthoracic echocardiogram (TTE), with often subsequent transesophageal echocardiogram (TEE) should be considered to evaluate presence of endocarditis/valvular involvement and to detect any implantable device infections, such as pacemaker or ICD lead vegetation.
Both CT scan and echocardiography have limitations due to interference from metallic components of the device. Therefore, if the extent of the infection and LVAD involvement is unclear and the suspicion for deep infection or embolic complications persists, a tagged white blood cell (WBC) scan may be of some utility in determining the source of infection. Unfortunately, tagged leukocyte scans have low sensitivity and low positive predictive value for diagnosing LVAD infections (11). Fluorine-18 fluorodeoxyglucose positron emission tomography integrated with computed tomography (18F FDG-PET-CT) has emerged as a promising modality to identify underling LVAD infections in patients presenting with non-localizing symptoms (12). This imaging can provide better resolution images than a WBC scan. In a recent meta-analysis (13), pooled sensitivity of FDG PET-CT for diagnosis of LVAD infection was 92% (95% CI: 82–97%) and specificity was 83% (95% CI: 24–99%). Obtaining PET-CT for suspected LVAD infection can be challenging as these are often only available to outpatients and insurance coverage can be an obstacle. Clinical judgement and exploratory surgery may be required if a peri-pump infection is suspected but is not visualized on imaging.
Equally important is isolation of causative microorganisms and in-vitro susceptibility testing to guide antimicrobial therapy for acute infection as well as chronic suppressive therapy, if indicated. At least two sets of blood, as well as wound cultures (when possible), should be obtained in all cases of suspected LVAD infection before starting any empiric antimicrobial therapy. In cases of chronic infection and negative bacterial cultures, mycobacterial and fungal cultures should be sent and use of broad range 16s Ribosomal RNA PCR may be considered in consultation with an infectious diseases specialist.
Management
Management of LVAD infection is challenging. Presence of hardware, that can be difficult or impossible to remove or exchange, poses a significant challenge to eliminating infection. Most LVAD associated infections are regarded as chronic in the absence of device removal. This is particularly true for deep soft tissue infections associated with the driveline, as well as pump and/or pocket infections. Patients may require prolonged antibiotic treatment, often administered intravenously, followed by antibiotic suppression in selected cases. Despite aggressive antimicrobial therapy, recurrences are common.
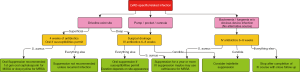
Management of the infection will largely depend on the extent of the infection, presence of deep soft tissue or LVAD pump/pocket infection, and causative organism (Figure 2).
Driveline infections
LVAD driveline exit site infections that are superficial, not associated with an abscess and do not cross fascia/muscle layer, may require only a short course of antibiotics. Depending on the causative organism, fourteen to twenty-eight days of antibiotic therapy, with option to use oral therapy, if in-vitro susceptibility permits, is recommended. Some patients can develop chronic LVAD driveline colonization and drainage. We do not recommend repeated or ongoing treatment of such drainage without other symptoms of active clinical infection, such as worsening erythema, fluctuance, or granulation tissue overgrowth. Repeated or prolonged antibiotic treatment for driveline exit site drainage does not eradicate indolent infection in most cases and can increase the risk of developing antibiotic resistance, limiting options of treatment when clinical infection does occur. The exception to this general rule is exit site infections caused by Methicillin-sensitive Staphylococcus aureus (MSSA). This organism is aggressive and is rarely controlled without antibiotic therapy. Resistance to beta lactam antibiotics, on the other hand, is very rare. Therefore, we recommend ongoing therapy for exit site infections due to MSSA with oral first generation cephalosporin antibiotics.
Pump/pocket infections
Deep soft tissue infections, or infections involving pump/pocket are treated with prolonged, often six to eight weeks or longer course of intravenous (IV) antibiotic therapy, followed by suppressive antibiotic therapy.
Surgery for LVAD infections
The role of surgery in management of LVAD-specific infections is not well studied. The conventional management strategy is to use antibiotics with or without surgery. Minor debridement is used for managing more superficial or mild LVAD driveline exit site infections, especially if an associated abscess is found. Patients who have severe, refractory driveline infections, with substantial exit site pain, and no mediastinal involvement, partial or total relocation of the driveline intra‐abdominally and wrapping with omentum can be considered for surgery. Risk of infection recurrence is still considerable, reported as 23% (14), but this could be considered as an option for patients who have substantial disability from the driveline infection and might decrease the need for recurrent admissions and subsequent prolonged hospital stay. While surgical management of driveline exit site infection is relatively straight forward, more complex surgery is usually required for patients who have deeper pump/pocket and/or mediastinal infection, often requiring plastic surgery involvement for skin defect closure, such as using omental flap for closure (15). LVAD exchange is typically reserved for severe cases that are unresponsive to or fail antibiotic therapy (16,17). However, the outcomes of such interventions, namely successful eradication of infection and mortality benefit after LVAD exchange, are not well understood. A systematic review and meta-analysis published in 2018 tried to answer these questions by reviewing 158 cases of CF‐LVAD‐specific infection and did not find a statistically significant difference in the overall mortality or infection recurrence rates (18). Infection recurrence rate post LVAD exchange was still quite high at around 27% at mean follow up of 290 days. In general, we recommend device exchange for highly resistant infections, especially if these infections affect the pump or pocket. We have had some success in complete device exchange (pump, cannulas, driveline) for patients with pump/pocket/cannula infections involving a multi drug resistant (MDR) organism.
Mueller and colleagues explored using a temporary endovascular left ventricular assist system as a bridge to definite device therapy after complete LVAD explantation in a patient with severe VAD pump/pocket infection and associated mediastinitis. The patient survived on temporary support for three months prior to re-implantation of HeartWare HVAD and remained infection free at one year follow up (19). However, as of now, there is no further evidence available beyond this case report to support this approach and therefore, it should not be considered as standard of care. It would be interesting to see more centers reporting their experience and survival rate using a similar approach.
Suppressive antibiotic therapy
The need for oral antibiotic suppression is not well studied. There are only a handful of case reports and case series published that address this subject, reporting failure rate from 29% to 32% in patients with chronic LVAD infection on oral suppressive therapy (20,21). We recommend chronic antibiotic suppression for infections that involve pump/pocket, as the risk of recurrent infection is thought to be high. Options for suppression largely depend on the causative organism and available choices of antibiotics. We recommend routine antibiotic suppression for all infections that are related to staphylococci (MRSA, MSSA, and coagulase negative staphylococci), as well as the majority of gram negative bacterial infections. Pseudomonas is an exception, given the concern for rapid development of resistance to the antibiotic that will be used, and overall limited options for antibiotic treatment. While oral antibiotic options are available in many cases, this might not always be possible in cases of resistant bacterial infections, or infections that fail oral antibiotic suppression and require continued IV therapy.
In cases when antibiotic suppression cannot be used, we suggest close surveillance, with consideration of surveillance blood cultures after initial antibiotic treatment is finished. Many patients with chronic infections, and patients who have been supported by LVADs for prolonged time, do not develop typical signs of sepsis early on, and can have bacteremia with minimal to no systemic symptoms.
There has been increasing interest in using bacteriophages as an adjunct therapy in patients who fail standard treatment with antibiotics with or without surgery, with some reports of successful eradication of refractory pump/pocket infections with local application of bacteriophage treatment (22). Using bacteriophages for treatment of prosthetic and/or LVAD infections is still considered to be an experimental approach, and much remains unknown about the feasibility and possible success rate of such an approach.
Outcomes
Infection is a serious complication of patients who are supported by LVAD, substantially changing their morbidity and mortality, as well as affecting quality of life. Recent IMACs registry analysis showed that overall survival of patients at twenty-four months after developing any infection was 59%, compared to 74.8% in patients who did not develop an infection. However, twenty-four-month overall survival after first VAD driveline and pocket infection differed substantially, with a survival of 75.7% in driveline infections that is close to the survival of patients who did not develop infection. In contrast, patients who developed pocket infections had much worse outcomes, with survival of 45.2% at twenty-four months. No data was reported on pump and/or cannula infections at twenty-four months, though eighteen-month survival was quite poor at 39% (4).
The impact LVAD infections have on clinical outcomes after transplantation is not well understood. Columbia-Presbyterian Medical Center experience showed that having a driveline infection during LVAD support predisposed patients to having an infection in the former LVAD pocket or driveline site after cardiac transplantation. However, pre-transplant device-related infection did not decrease post-transplant survival at one year. No other LVAD-related infection, including pocket infection, was associated with post-transplant infections (23). A systematic review and meta-analysis examined post-transplant survival of patients with established LVAD-specific infection. The analysis showed worse outcomes for patients who had pre-existing LVAD infection prior to transplantation, however the sample size did not allow for differentiation between driveline and deeper pump/pocket infections, and impact on outcomes (24). The authors noted that the reason for the worse outcomes was not clear, and could be related to sensitization and acute rejection events after transplantation, as well as renal dysfunction and overall frailty. The most recently published retrospective review of patients with LVAD-specific and related infections at Mayo Clinic studied their post heart transplant outcomes. LVAD-specific infections were treated with two weeks of pathogen-directed therapy post heart transplant without any relapses (25).
Conclusions
CF-LVAD infections are challenging to diagnose and treat, with little evidence to guide best diagnostic and management strategies. With early identification of the infection and aggressive medical, and sometimes surgical management, a significant proportion of infections can be cured, or at least successfully suppressed. The role of LVAD exchange in curing infection is unclear. The ultimate cure of LVAD infection is heart transplantation in eligible patients, with favorable outcomes.
Elimination of the driveline would certainly resolve the major risk factor for development of LVAD infections. The challenge in creating such a device is its high energy requirement. Transcutaneous energy transmission systems (TETS) continue to be explored as a potential method to power implantable cardiac devices, however their limitations include thermal injury from the external coil, as well as range and alignment problems that can reduce energy transfer. With TETS, patients are still required to wear portable batteries (26). Free-range resonant electrical energy delivery system (FREE-D) is another mode of power transfer under investigation. This technology enables wireless power transfer to an implanted cardiac device without need for direct contact between the patient and the energy source (26). The problem of creating a lifelong implantable cardiac device that does not require an external energy source has yet to be solved (27).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fang J, Mensah GA, Croft JB, et al. Heart Failure-Related Hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol 2008;52:428-34. [Crossref] [PubMed]
- Gillum RF. Epidemiology of heart failure in the United States. Am Heart J 1993;126:1042-7. [Crossref] [PubMed]
- Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2018 Annual Data Report: Heart. Am J Transplant 2020;20:340-426. [Crossref] [PubMed]
- Hannan MM, Xie R, Cowger J, et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2019;38:364-73. [Crossref] [PubMed]
- Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 2011;30:375-84. [Crossref] [PubMed]
- Tattevin P, Flécher E, Auffret V, et al. Risk factors and prognostic impact of left ventricular assist device–associated infections. Am Heart J 2019;214:69-76. [Crossref] [PubMed]
- Pavlovic NV, Randell T, Madeira T, et al. Risk of left ventricular assist device driveline infection: A systematic literature review. Heart Lung 2019;48:90-104. [Crossref] [PubMed]
- Kusne S, Mooney M, Danziger-Isakov L, et al. An ISHLT consensus document for prevention and management strategies for mechanical circulatory support infection. J Heart Lung Transplant 2017;36:1137-53. [Crossref] [PubMed]
- Martin BJ, Luc JGY, Maruyama M, et al. Driveline site is not a predictor of infection after ventricular assist device implantation. ASAIO J 2018;64:616-22. [Crossref] [PubMed]
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017;376:451-60. [Crossref] [PubMed]
- de Vaugelade C, Mesguich C, Nubret K, et al. Infections in patients using ventricular-assist devices: Comparison of the diagnostic performance of 18 F-FDG PET/CT scan and leucocyte-labeled scintigraphy. J Nucl Cardiol 2019;26:42-55. [Crossref] [PubMed]
- ten Hove D, Treglia G, Slart RHJA, et al. The value of 18F-FDG PET/CT for the diagnosis of device-related infections in patients with a left ventricular assist device: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021;48:241-53. [Crossref] [PubMed]
- Tam MC, Patel VN, Weinberg RL, et al. Diagnostic Accuracy of FDG PET/CT in Suspected LVAD Infections: A Case Series, Systematic Review, and Meta-Analysis. JACC Cardiovasc Imaging 2020;13:1191-202. [Crossref] [PubMed]
- Pieri M, Scandroglio AM, Müller M, et al. Surgical management of driveline infections in patients with left ventricular assist devices. J Card Surg 2016;31:765-771. [Crossref] [PubMed]
- Pieri M, Müller M, Scandroglio AM, et al. Surgical Treatment of Mediastinitis with Omentoplasty in Ventricular Assist Device Patients: Report of Referral Center Experience. ASAIO J 2016;62:666-70. [Crossref] [PubMed]
- Pereda D, Conte J V. Left ventricular assist device driveline infections. Cardiol Clin 2011;29:515-27. [Crossref] [PubMed]
- Schmack B, Sabashnikov A, Weymann A, et al. Left ventricular assist devices exchange: Why, when and how to do it — Experience from experts. J Thorac Dis 2019;11:S963-6. [Crossref] [PubMed]
- Bauer TM, Choi JH, Luc JGY, et al. Device exchange versus nonexchange modalities in left ventricular assist device-specific infections: A systematic review and meta-analysis. Artif Organs 2019;43:448-57. [Crossref] [PubMed]
- Mueller M, Potapov E, Krabatsch T. Usefulness of a temporary endovascular left ventricular assist system as a bridge to facilitate treatment of mediastinitis associated with a permanent device. J Heart Lung Transplant 2019;38:476-8. [Crossref] [PubMed]
- Jennings DL, Chopra A, Chambers R, Morgan JA. Clinical outcomes associated with chronic antimicrobial suppression therapy in patients with continuous-flow left ventricular assist devices. Artif Organs 2014;38:875-9. [Crossref] [PubMed]
- Radcliffe C, Doilicho N, Niu YS, Grant M. Efficacy and safety of chronic antimicrobial suppression therapy for left ventricular assist device driveline infections: A single-center descriptive experience. Transpl Infect Dis 2020;22:e13379 [Crossref] [PubMed]
- Mulzer J, Trampuz A, Potapov E V. Treatment of chronic left ventricular assist device infection with local application of bacteriophages. Eur J Cardiothorac Surg 2020;57:1003-4. [Crossref] [PubMed]
- Schulman AR, Martens TP, Russo MJ, et al. Effect of Left Ventricular Assist Device Infection on Post-transplant Outcomes. J Heart Lung Transplant 2009;28:237-42. [Crossref] [PubMed]
- Chahal D, Sepehry AA, Nazzari H, et al. The Impact of Left Ventricular Assist Device Infections on Postcardiac Transplant Outcomes: A Systematic Review and Meta-Analysis. ASAIO J 2019;65:827-36. [Crossref] [PubMed]
- Garrigos ZE, Castillo Almeida NE, Gurram P, et al. Management and outcome of left ventricular assist device infections in patients undergoing cardiac transplantation. Open Forum Infect Dis 2020;7:ofaa303.
- Bleszynski PA, Luc JGY, Schade P, et al. Current State and Future Perspectives of Energy Sources for Totally Implantable Cardiac Devices. ASAIO J 2016;62:639-45. [Crossref] [PubMed]
- Tchantchaleishvili V, Phillips SJ. Update in Artificial Heart Technology: Are We There Yet? Artif Organs 2016;40:1099-100. [Crossref] [PubMed]