Technical pitfalls and solutions in extrapleural pneumonectomy
Introduction
Extrapleural pneumonectomy (EPP) requires unique surgical techniques and peri-operative management. Over the past 20 years, meticulous patient selection, careful intra-operative conduct of the operation and diligent post-operative management have reduced the mortality of this operation to less than 5% in experienced centers, even after induction therapy (1-3).
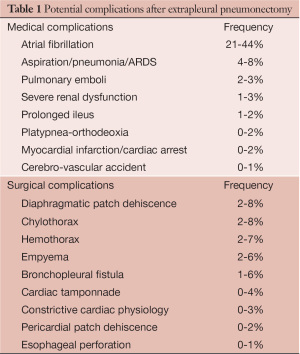
EPP carries a high rate of peri-operative complication, hence prevention, as well as early recognition of potential problems, is important to minimize the morbidity of this operation. Occasionally, early reoperation has to be conducted to limit the risk of further complications leading to a non-salvageable situation. A list of the most frequent complications following EPP based on our experience and after review of the literature is summarized in Table 1 (1,4-7).
Full table
Atrial fibrillation is by far the most common complication after EPP. Other frequent medical complications include infectious/inflammatory lung injury and venous thromboembolic events (VTE). Vocal cord palsy related to intra-operative recurrent nerve injury, postoperative ileus and somnolence are risk factors for aspiration and pneumonia that should be carefully monitored. VTE prophylaxis during hospital stay and for at least 4 to 6 weeks after discharged is important (8,9).
The most common surgical complications include diaphragmatic patch dehiscence, chylothorax, hemothorax and empyema with or without bronchopleural fistula (BPF). In this article, we will describe three clinical scenarios with specific surgical complications that we encountered. Mechanism of their occurrence as well as recommendations for early diagnosis and management will be highlighted. Strategies to avoid these complications will also be emphasized.
Diaphragmatic patch dehiscence
Clinical scenario
A 52 year old man underwent an EPP using our standard approach that includes resection of the 5th or 6th rib associated with a second thoracotomy in the 8th intercostal space performed through the same skin incision (Figure 1). The second lower incision greatly facilitates dissection of the diaphragm along its posterior and lateral attachment. This incision also provides a direct view to the inferior vena cava (IVC), phrenic veins and hepatic veins from underneath the diaphragm. The diaphragm can then be sharply dissected off the IVC and the phrenic veins ligated. The diaphragm is then repaired with 1 mm Gore-Tex mesh (W.L. Gore and Associates, Flagstaff, AZ) through the second incision.
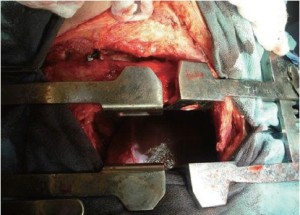
This patient was planned for adjuvant hemi-thoracic radiation of 50-60 Gy and therefore the diaphragmatic mesh was placed under tension using a single patch in anatomic position to facilitate the postoperative radiation. Care was taken to avoid any constriction of the IVC when the mesh was positioned. Occasionally, a split of the diaphragmatic mesh along the IVC is performed to ensure that the mesh does not cause any stenotic string along the IVC.
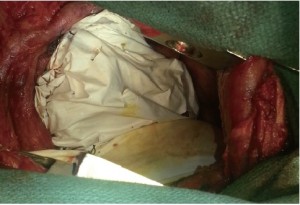
The pericardial mesh (pericardial membrane, Gore-Tex mesh, W.L. Gore and Associates, Flagstaff, AZ) was then fixed with interrupted stitches on the anterior and posterior pericardial edge. The pericardial mesh was purposely kept loose to avoid any postoperative constriction (Figure 2). Good hemostasis was achieved by the end of surgery.
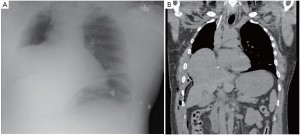
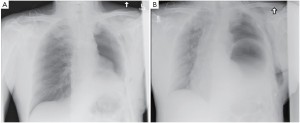
The patient had an uneventful surgery and was transferred to the recovery room where he was extubated. A chest X-ray performed before extubation demonstrated satisfactory positioning of the diaphragmatic and pericardial meshes. The patient remained well overnight. The next chest X-ray performed on the first postoperative day demonstrated a convex opacity in the right lower chest (Figure 3). This finding raised the possibility of herniation of the liver. A computed tomography (CT) scan was therefore urgently performed, confirming the diagnosis. Liver enzymes were also increased (AST of 1,981 U/L and ALT of 1,726 U/L) as a consequence of liver strangulation. The patient was immediately taken back to the operating room to reposition the liver and the mesh, which had dehisced posteriorly. Postoperatively, he was extubated after completion of the surgery and made an uneventful recovery. His liver enzymes normalized. He underwent adjuvant hemi-thoracic radiation starting 5 weeks after surgery.
Diagnosis and management
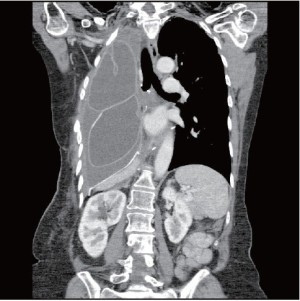
Mesh dehiscence is a rare but well described complication after EPP. It is reported in 2% to 8% in large surgical series (1,4,5). Early diagnosis and rapid re-intervention is crucial to preclude any additional problem related to the dehiscence. The clinical presentation can be subtle and the diagnosis only suspected on a chest X-ray. Partial dehiscence may lead to strangulation of the liver, stomach, or small bowel, while large dehiscence can lead to inadequate clearance of the secretions due to the paradoxical movement of the abdomen into the chest (Figure 4). Early signs and symptoms can include systemic hypotension, hypoxemia, abdominal pain and a weak cough. Clinicians should keep a high index of suspicion and repeat chest X-ray should be performed when any of these signs or symptoms is present. An abnormal chest X-ray with an unexplained opacity in the lower pleural space should lead to a CT scan and/or chest reopening if the index of suspicion is high. Unrecognized dehiscence of the diaphragmatic mesh may lead to major hemodynamic problem, ischemic organ, and sepsis if it is not promptly recognized.
Prevention
Our radiation protocol has changed over the past 4 years. We currently deliver pre-operative radiation therapy using a course of hypofractionated hemi-thoracic intensity modulated radiation therapy (IMRT) for a total of 25 to 30 Gy in 5 fractions that is completed about a week before EPP (10). Since no adjuvant radiation is required, we do not place the diaphragmatic mesh under tension in anatomic position anymore, thus limiting the risk of dehiscence.
We use two 1 mm Gore-Tex mesh that are stapled together with two 60 mm load of blue TA staplers (Covidien, Mansfield, MA) using a similar technique described by Sugarbaker and colleagues (1). The stitches to fix the diaphragmatic mesh are placed in the intercostal space, pericardium and periosteum of the vertebral body or intervebral disc to have adequate anchoring points for fixation. The stitches are currently placed above the rim of the diaphragm to facilitate access. The diaphragmatic rim does not have adequate strength and should not be used to secure the mesh. The posterior fixation of the mesh can occasionally be difficult due to the lack of adequate structure to anchor the mesh. We therefore always place one stitch in the periosteum of the vertebral body and/or intervertebral disc to have at least one good anchoring point posteriorly. The periosteum of the vertebral body and intervertebral disc are exposed at the desired level (typically T8-T9) by dissecting the prevertebral longitudinal ligament and intercostal arteries running in front of the spine at that level to prevent any bleeding.
Chylothorax
Clinical scenario
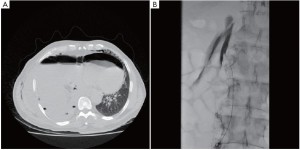
A 64 year old man with right sided biphasic mesothelioma underwent an EPP after induction hemi-thoracic hypofractionated IMRT. The surgery was uneventful. The thoracic duct was ligated in the lower chest just above the diaphragmatic crus. Postoperatively, the chest tube drainage was higher than usual at an average of 80-100 mL/hr. After 24 hours, the patient was started on a clear fluid diet. The pleural fluid was examined for chylomicrons to rule out a chylothorax since the pleural drainage remained over 1 liter per day. Chylomicrons were absent and the chest tube was removed. Within 24 hours of chest tube removal, the patient developed low blood pressure and significant subcutaneous emphysema. An urgent CT scan was performed (Figure 5). A chest tube was immediately reinserted draining large amount of chylous fluid. The patient’s blood pressure improved and all parameters rapidly normalized. The patient had developed a tension chylothorax and was therefore taken back to the operating room the following day after giving him 50 cc of cream to drink 2 hours before the surgery. The thoracotomy was reopened and the diaphragmatic mesh was detached posteriorly to assess the leak. We found a large chyle leak in the retroperitoneum below the site of the previous thoracic duct ligation. Due to the large leak located below the diaphragmatic crus, the success of the second ligation was limited and the patient was maintained on a strict fasting diet with parenteral nutrition for 7 days along with octreotide injection subcutaneously (Sandostatin, Norvartis, Mississauga, Canada). A chest tube was maintained in place, but despite these measures the chyle leak persisted at a high rate. After discussion with the interventional radiologists, we elected to proceed with an embolization of the cisterna chyli through a percutaneous transabdominal approach. After advancing the needle into the cisterna chyli, a lymphangiogram was performed demonstrating the leak at the top of the cisterna chyli (Figure 5). The cisterna chyli could not be adequately embolized, but fortunately, the lymphangiogram itself created sufficient viscosity within the lymphatic drainage that the leak stopped. The patient then resumed a normal diet over the following few days without any evidence of chyle leak. The chest tube was removed and he was discharged home in good condition.
Diagnosis and management
Chylothorax occurs between 2% and 8% of patients after EPP (1,4-6). Occasionally, complete macroscopic resection of right sided bulky tumor requires deep resection along the diaphragmatic crus leading to a higher risk of damaging the thoracic duct. As seen in this patient, routine ligation of the thoracic duct may not be sufficient to prevent this problem and early recognition is important.
The management of the chylothorax after EPP can be difficult. Our preferred approach is to treat it surgically by taking the patient back to the operating room once the diagnosis is made. Conservative treatment has generally failed in our experience. In this case the leak was located at the upper level of the cisterna chyli and the thoracic approach did not provide adequate access to the leak. A transabdominal approach was therefore required to control the leak. We chose a transabdominal percutaneous approach that has been described by a group in Philadelphia (11). This approach has been very successful in their experience and that of others, but is difficult since it requires a percutaneous access to the cisterna chyli by advancing the needle through the small bowel and few interventional radiologists have experience with this technique (11,12). The alternative to the percutaneous embolization would have been a laparoscopic approach to access the leak below the diaphragmatic crus (13).
Prevention
Some authors advocate routine ligation of the thoracic duct during EPP to limit the risk of chyle leak. In our practice, we do ligate the thoracic duct only when we have concern about possible damage to the duct. In this clinical scenario, although the thoracic duct was ligated at the time of EPP, the leak occurred below the ligature and ligation of the thoracic duct may have in fact exacerbated the leak due to the upstream occlusion.
Bronchopleural fistula
Clinical scenario
A 61 year old woman underwent a right sided EPP after induction chemotherapy. The surgery was uneventful. The bronchial stump was stapled as per our routine. The stump was not covered. Postoperatively, the patient was discharged home after 12 days without any postoperative complications. She then went on to start her hemi-thoracic radiation 4 weeks after the surgery. Three days after starting her radiation, she developed a productive cough with expectoration of clear yellowish sputum that was exacerbated when she was lying flat. A chest X-ray demonstrated a drop in the air-fluid level. A chest tube was immediately placed and a bronchoscopy confirmed the presence of a small BPF.
The patient was started on antibiotics and taken to the operating room the same day with a plan to perform a thoracic window. To our surprise, the pleural cavity was clean with no evidence of empyema. A small fistula was detected in the upper corner of the bronchial stump. Since the pleural cavity was clean, we decided to close the fistula with a muscle flap. The pectoralis major muscle was therefore elevated on its pedicle and transferred into the pleural cavity through the second intercostal space. The muscle reached the pulmonary hilum without any tension. Several stitches of 4-0 PDS (Ethicon, Somerville, N.J.) were used to attach the flap to the edge of the bronchial stump. We then filled the pleural cavity with 2 breast implants inflated with normal saline to maintain the flap in good position against the stump. There was no air leak from the bronchial stump and the chest was closed in a standard fashion without drainage. The patient rapidly recovered. She resumed her adjuvant radiation therapy 2 days later and completed her course to 50 Gy as planned. She has not developed any recurrence of her BPF or any empyema after more than 18 months of follow-up (Figure 6).
Diagnosis and management
BPF after EPP is a dreadful complication. BPF typically occurs on the right side. Treatment usually involves a Clagett window with removal of the pericardial and diaphragmatic meshes at the same time or in a staged fashion (1,4). Schneiter and colleagues from Zurich in Switzerland have described an accelerated approach that involves repeated pleural debridement in the operating room every 2 days with gentle chest tube suction to clear the empyema before permanently closing the thoracotomy (14). The BPF is sealed during the process with a muscle flap or the omentum. This approach was successful in the vast majority of their patients even after EPP (5).
Prevention
In order to limit the risk of BPF, the bronchial stump should be covered at the time of surgery. This practice has become our routine since we started our protocol of induction hemi-thoracic radiation 4 years ago. The bronchial stump coverage on the left side is usually not a problem. On the right side, we have used the posterior pericardium, the thymus or the omentum to cover the bronchial stump. The posterior pericardium can be dissected off the superior vena cava (SVC) and the right pulmonary artery. This mobilization typically provides enough mobility for the posterior pericardium to cover the stump and to attach it to the peribronchial tissue. Alternately, the thymus is mobilized and advanced underneath the SVC to cover the stump. Care should be taken not to open the left pleural space or damage the left innominate vein when mobilizing the thymus. The third option is the omentum, which can be mobilized through the thoracotomy and usually will reach the pulmonary hilum without difficulty.
Conclusions
EPP is an extensive operation that can currently be performed with limited mortality. However, the risk of medical and surgical complications remains high. Hence, prevention, early recognition and adequate management of complications such as diaphragmatic patch dehiscence, chylothorax, and empyema with or without BPF are essential for good outcomes.
Acknowledgements
Stéphane Collaud MD, MSc was supported for this work by the “American Association for Thoracic Surgery’s Evarts A. Graham Memorial Traveling Fellowship”.
Disclosure: The authors declare no conflict of interest.
References
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46.
- Opitz I, Tagawa T, Friess M, et al. Peri-operative outcome of extrapleural pneumonectomy after chemotherapy for malignant pleural mesothelioma in 189 patients from two institutions. The 11th International Conference of the International Mesothelioma Interest Group. Boston, MA, 2012.
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13.
- de Perrot M, McRae K, Anraku M, et al. Risk factors for major complications after extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Thorac Surg 2008;85:1206-10.
- Opitz I, Kestenholz P, Lardinois D, et al. Incidence and management of complications after neoadjuvant chemotherapy followed by extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2006;29:579-84.
- Stewart DJ, Martin-Ucar AE, Edwards JG, et al. Extra-pleural pneumonectomy for malignant pleural mesothelioma: the risks of induction chemotherapy, right-sided procedures and prolonged operations. Eur J Cardiothorac Surg 2005;27:373-8.
- Zellos L, Jaklitsch MT, Al-Mourgi MA, et al. Complications of extrapleural pneumonectomy. Semin Thorac Cardiovasc Surg 2007;19:355-9.
- Patel A, Anraku M, Darling GE, et al. Venous thromboembolism in patients receiving multimodality therapy for thoracic malignancies. J Thorac Cardiovasc Surg 2009;138:843-8.
- Billè A, Okiror L, Karenovics W, et al. What is the optimum strategy for thromboembolic prophylaxis following extrapleural pneumonectomy in patients with malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2012;15:201-3.
- de Perrot M, Opitz I, Anraku M, et al. Results of short accelerated hypofractionated hemithoracic intensity modulated radiation therapy followed by extrapleural pneumonectomy for malignant pleural mesothelioma. The 11th International Conference of the International Mesothelioma Interest Group. Boston, MA, 2012.
- Itkin M, Chen EH. Thoracic duct embolization. Semin Intervent Radiol 2011;28:261-6.
- Nadolski GJ, Itkin M. Thoracic duct embolization (TDE) for non-traumatic chylous effusion: Experience in 34 patients. Chest 2012. [Epub ahead of print].
- Tsubokawa N, Hamai Y, Hihara J, et al. Laparoscopic thoracic duct clipping for persistent chylothorax after extrapleural pneumonectomy. Ann Thorac Surg 2012;93:e131-2.
- Schneiter D, Grodzki T, Lardinois D, et al. Accelerated treatment of postpneumonectomy empyema: a binational long-term study. J Thorac Cardiovasc Surg 2008;136:179-85.





